RPC4046, A Novel Anti-interleukin-13 Antibody, Blocks IL-13 Binding to IL-13 α1 and α2 Receptors: A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation First-in-Human Study
- PMID: 28455782
- PMCID: PMC5487860
- DOI: 10.1007/s12325-017-0525-8
RPC4046, A Novel Anti-interleukin-13 Antibody, Blocks IL-13 Binding to IL-13 α1 and α2 Receptors: A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation First-in-Human Study
Abstract
Introduction: A unique anti-interleukin (IL)-13 monoclonal antibody, RPC4046, was generated on the basis of differential IL-13 receptor (R) blockade as assessed in a murine asthma model; the safety, tolerability, pharmacokinetics, and pharmacodynamics of RPC4046 were evaluated in a first-in-human study.
Methods: Anti-IL-13 antibodies with varying receptor blocking specificity were evaluated in the ovalbumin-induced murine asthma model. A randomized, double-blind, placebo-controlled, dose-escalation first-in-human study (NCT00986037) was conducted with RPC4046 in healthy adults and patients with mild to moderate controlled asthma.
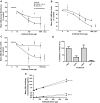
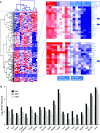
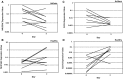
Results: In the ovalbumin model, blocking IL-13 binding to both IL-13Rs (IL-13Rα1 and IL-13Rα2) inhibited more asthma phenotypic features and more fully normalized the distinct IL-13 gene transcription associated with asthma compared with blocking IL-13Rα1 alone. In humans, RPC4046 exposure increased dose-dependently; pharmacokinetics were similar in healthy and asthmatic subjects, and blockade of both IL-13Rs uniquely affected IL-13 gene transcription. A minority of participants (28%) had antidrug antibodies, which were transient and appeared not to affect pharmacokinetics. Adverse event profiles were similar in healthy and asthmatic subjects, without dose-related or administration route differences, systemic infusion-related reactions, or asthma symptom worsening. Adverse events were mild to moderate, with none reported as probably related to RPC4046 or leading to discontinuations. Non-serious upper respiratory tract infections were more frequent with RPC4046 versus placebo.
Conclusion: RPC4046 is a novel anti-IL-13 antibody that blocks IL-13 binding to both receptors and more fully blocks the asthma phenotype. These results support further investigation of RPC4046 for IL-13-related allergic/inflammatory diseases (e.g., asthma and eosinophilic esophagitis).
Funding: AbbVie Inc. sponsored the studies and contributed to the design and conduct of the studies, data management, data analysis, interpretation of the data, and in the preparation and approval of the manuscript.
Keywords: Asthma; IL-13; Respiratory.
Figures

References
-
- Luttmann W, Knoechel B, Foerster M, Matthys H, Virchow JC, Jr, Kroegel C. Activation of human eosinophils by IL-13. Induction of CD69 surface antigen, its relationship to messenger RNA expression, and promotion of cellular viability. J Immunol. 1996;157:1678–1683. - PubMed
-
- Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–2694. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

