Galectin-1 expression imprints a neurovascular phenotype in proliferative retinopathies and delineates responses to anti-VEGF
- PMID: 28455954
- PMCID: PMC5464805
- DOI: 10.18632/oncotarget.17129
Galectin-1 expression imprints a neurovascular phenotype in proliferative retinopathies and delineates responses to anti-VEGF
Abstract
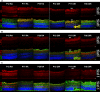
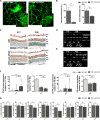
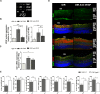
Neovascular retinopathies are leading causes of irreversible blindness. Although vascular endothelial growth factor (VEGF) inhibitors have been established as the mainstay of current treatment, clinical management of these diseases is still limited. As retinal impairment involves abnormal neovascularization and neuronal degeneration, we evaluated here the involvement of galectin-1 in vascular and non-vascular alterations associated with retinopathies, using the oxygen-induced retinopathy (OIR) model. Postnatal day 17 OIR mouse retinas showed the highest neovascular profile and exhibited neuro-glial injury as well as retinal functional loss, which persisted until P26 OIR. Concomitant to VEGF up-regulation, galectin-1 was highly expressed in P17 OIR retinas and it was mainly localized in neovascular tufts. In addition, OIR induced remodelling of cell surface glycophenotype leading to exposure of galectin-1-specific glycan epitopes. Whereas VEGF returned to baseline levels at P26, increased galectin-1 expression persisted until this time period. Remarkably, although anti-VEGF treatment in P17 OIR improved retinal vascularization, neither galectin-1 expression nor non-vascular and functional alterations were attenuated. However, this functional defect was partially prevented in galectin-1-deficient (Lgals1-/-) OIR mice, suggesting the importance of targeting both VEGF and galectin-1 as non-redundant independent pathways. Supporting the clinical relevance of these findings, we found increased levels of galectin-1 in aqueous humor from patients with proliferative diabetic retinopathy and neovascular glaucoma. Thus, using an OIR model and human samples, we identified a role for galectin-1 accompanying vascular and non-vascular retinal alterations in neovascular retinopathies.
Keywords: galectin-1; neovascularization; neurodegeneration; retinopathies; vascular endothelial growth factor.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203. doi: 10.1056/NEJMoa1414264. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

