TrkC promotes colorectal cancer growth and metastasis
- PMID: 28455963
- PMCID: PMC5522271
- DOI: 10.18632/oncotarget.17289
TrkC promotes colorectal cancer growth and metastasis
Abstract
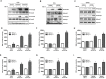
The current work reveals that TrkC receptor is crucial to many aspects of tumorigenicity and metastasis of cancer. However, with only a few exceptions, such as colorectal cancer (CRC), where suppressing tumorigenic and metastatic ability via expression of TrkC as tumor suppressor have been proposed. These diverse lines of evidence led us to investigate whether TrkC is involved in CRC progression. By using mouse models and molecular biology analyses, we demonstrate that TrkC acts as an activator in tumorigenicity and metastasis of colorectal cancer. In this study, TrkC was frequently overexpressed in CRC cells, patients' tumor samples and an azoxymethane/dextran sulphate sodium-induced mouse model of colitis-associated CRCs. TrkC expression was associated with a high-grade CRC phenotype, leading to significantly poorer survival. Also, TrkC expression promoted the acquisition of motility and invasiveness in CRC. Moreover, TrkC increased the ability to form tumor spheroids, a property associated with cancer stem cells. Importantly, knockdown of TrkC in malignant mouse or human CRC cells inhibited tumor growth and metastasis in a mouse xenograft model. Furthermore, TrkC enhanced metastatic potential and induced proliferation by aberrant gain of AKT activation and suppression of transforming growth factor (TGF)-β signalling. Interestingly, TrkC not only modulated the actions of TGF-β type II receptor, but also attenuated expression of this receptor. These findings reveal an unexpected physiological role of TrkC in the pathogenesis of CRC. Therefore, TrkC is a potential target for designing effective therapeutic strategies for CRC development.
Keywords: EMT program; TrkC; colorectal cancer; metastasis; tumorigenicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Jin W, Lee JJ, Kim MS, Son BH, Cho YK, Kim HP. DNA methylation-dependent regulation of TrkA, TrkB, and TrkC genes in human hepatocellular carcinoma. Biochem Biophys Res Commun. 2011;406:89–95. - PubMed
-
- Jin W, Kim GM, Kim MS, Lim MH, Yun C, Jeong J, Nam JS, Kim SJ. TrkC plays an essential role in breast tumor growth and metastasis. Carcinogenesis. 2010;31:1939–47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

