Incorporation of the Kaposi's sarcoma-associated herpesvirus capsid vertex-specific component (CVSC) into self-assembled capsids
- PMID: 28456575
- PMCID: PMC5661877
- DOI: 10.1016/j.virusres.2017.04.016
Incorporation of the Kaposi's sarcoma-associated herpesvirus capsid vertex-specific component (CVSC) into self-assembled capsids
Abstract
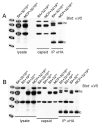
Self-assembly of herpesvirus capsids can be accomplished in heterologous expression systems provided all six capsid proteins are present. We have demonstrated the assembly of icosahedral Kaposi's sarcoma-associated herpesvirus (KSHV) capsids in insect cells using the baculovirus expression system. Using this self-assembly system we investigated whether we could add additional capsid associated proteins and determine their incorporation into the assembled capsid. We chose the capsid vertex-specific component (CVSC) proteins encoded by open reading frames (ORFs) 19 and 32 to test this. This complex sits on the capsid vertex and is important for capsid maturation in herpesvirus-infected cells. Co-immunoprecipitation assays were used to initially confirm a bi-molecular interaction between ORF19 and ORF32. Both proteins also precipitated the triplex proteins of the capsid shell (ORF26 and ORF62) as well as the major capsid protein (ORF25). Capsid immunoprecipitation assays revealed the incorporation of ORF19 as well as ORF32 into assembled capsids. Similar experiments also showed that the incorporation of each protein occurred independent of the other. These studies reveal biochemically how the KSHV CVSC interacts with the capsid shell.
Keywords: Capsid; Capsid incorporation; Capsid vertex-specific component; KSHV; Major capsid protein; ORF19; ORF32; ORF69; Triplex proteins.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures


References
-
- Baines JD. Herpes simplex virus capsid assembly and DNA packaging: a present and future antiviral drug target. Trends Microbiol. 2011;19(12):606–613. - PubMed
-
- Borst EM, Bauerfeind R, Binz A, Stephan TM, Neuber S, Wagner K, Steinbruck L, Sodeik B, Lenac Rovis T, Jonjic S, Messerle M. The Essential Human Cytomegalovirus Proteins pUL77 and pUL93 Are Structural Components Necessary for Viral Genome Encapsidation. J Virol. 2016;90(13):5860–5875. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

