Optimization of the Production Process and Characterization of the Yeast-Expressed SARS-CoV Recombinant Receptor-Binding Domain (RBD219-N1), a SARS Vaccine Candidate
- PMID: 28456726
- PMCID: PMC5612335
- DOI: 10.1016/j.xphs.2017.04.037
Optimization of the Production Process and Characterization of the Yeast-Expressed SARS-CoV Recombinant Receptor-Binding Domain (RBD219-N1), a SARS Vaccine Candidate
Abstract
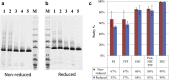
From 2002 to 2003, a global pandemic of severe acute respiratory syndrome (SARS) spread to 5 continents and caused 8000 respiratory infections and 800 deaths. To ameliorate the effects of future outbreaks as well as to prepare for biodefense, a process for the production of a recombinant protein vaccine candidate is under development. Previously, we reported the 5 L scale expression and purification of a promising recombinant SARS vaccine candidate, RBD219-N1, the 218-amino acid residue receptor-binding domain (RBD) of SARS coronavirus expressed in yeast-Pichia pastoris X-33. When adjuvanted with aluminum hydroxide, this protein elicited high neutralizing antibody titers and high RBD-specific antibody titers. However, the yield of RBD219-N1 (60 mg RBD219-N1 per liter of fermentation supernatant; 60 mg/L FS) still required improvement to reach our target of >100 mg/L FS. In this study, we optimized the 10 L scale production process and increased the fermentation yield 6- to 7-fold to 400 mg/L FS with purification recovery >50%. A panel of characterization tests indicated that the process is reproducible and that the purified, tag-free RBD219-N1 protein has high purity and a well-defined structure and is therefore a suitable candidate for production under current Good Manufacturing Practice and future phase-1 clinical trials.
Keywords: Pichia pastoris; circular dichroism; hydrophobic interaction chromatography; protein characterization; protein purification.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Jaume M., Yip M.S., Kam Y.W. SARS CoV subunit vaccine: antibody-mediated neutralisation and enhancement. Hong Kong Med J. 2012;18 Suppl 2:31–36. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

