Methods, Tools and Current Perspectives in Proteogenomics
- PMID: 28456751
- PMCID: PMC5461547
- DOI: 10.1074/mcp.MR117.000024
Methods, Tools and Current Perspectives in Proteogenomics
Abstract
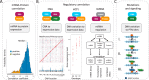
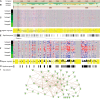
With combined technological advancements in high-throughput next-generation sequencing and deep mass spectrometry-based proteomics, proteogenomics, i.e. the integrative analysis of proteomic and genomic data, has emerged as a new research field. Early efforts in the field were focused on improving protein identification using sample-specific genomic and transcriptomic sequencing data. More recently, integrative analysis of quantitative measurements from genomic and proteomic studies have identified novel insights into gene expression regulation, cell signaling, and disease. Many methods and tools have been developed or adapted to enable an array of integrative proteogenomic approaches and in this article, we systematically classify published methods and tools into four major categories, (1) Sequence-centric proteogenomics; (2) Analysis of proteogenomic relationships; (3) Integrative modeling of proteogenomic data; and (4) Data sharing and visualization. We provide a comprehensive review of methods and available tools in each category and highlight their typical applications.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

