NIH workshop report on the trans-agency blood-brain interface workshop 2016: exploring key challenges and opportunities associated with the blood, brain and their interface
- PMID: 28457227
- PMCID: PMC5410699
- DOI: 10.1186/s12987-017-0061-6
NIH workshop report on the trans-agency blood-brain interface workshop 2016: exploring key challenges and opportunities associated with the blood, brain and their interface
Abstract
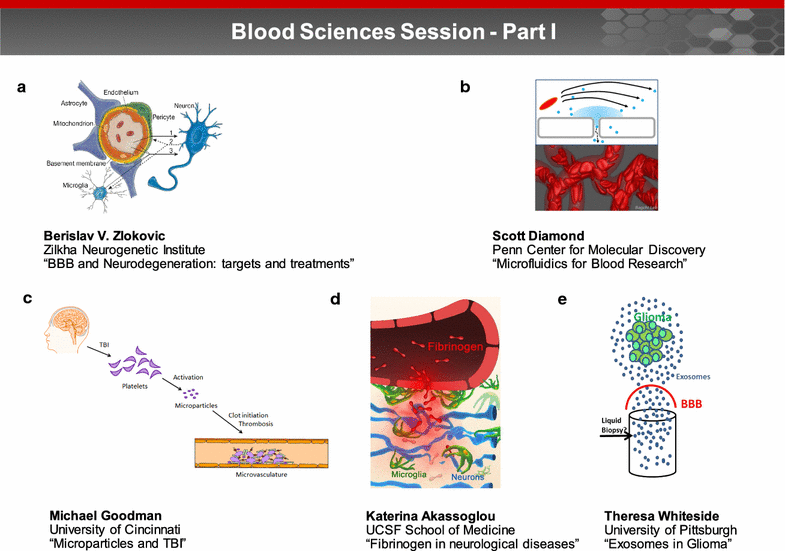
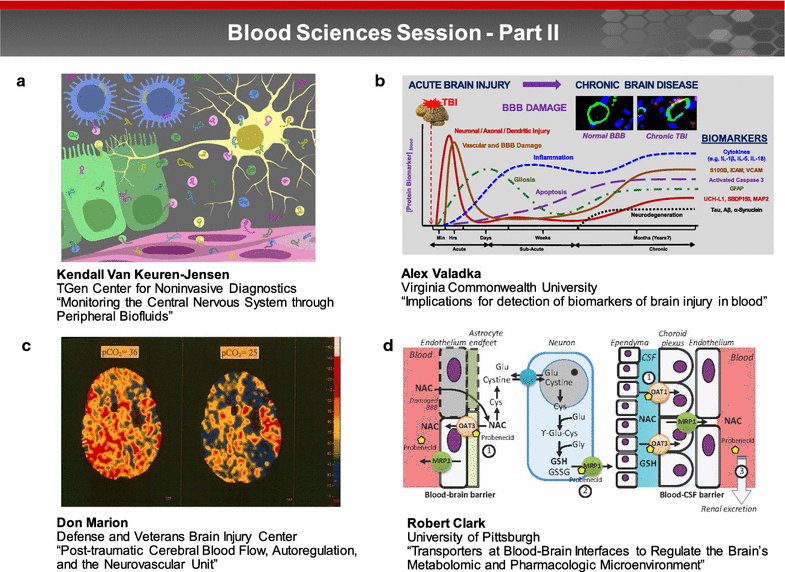
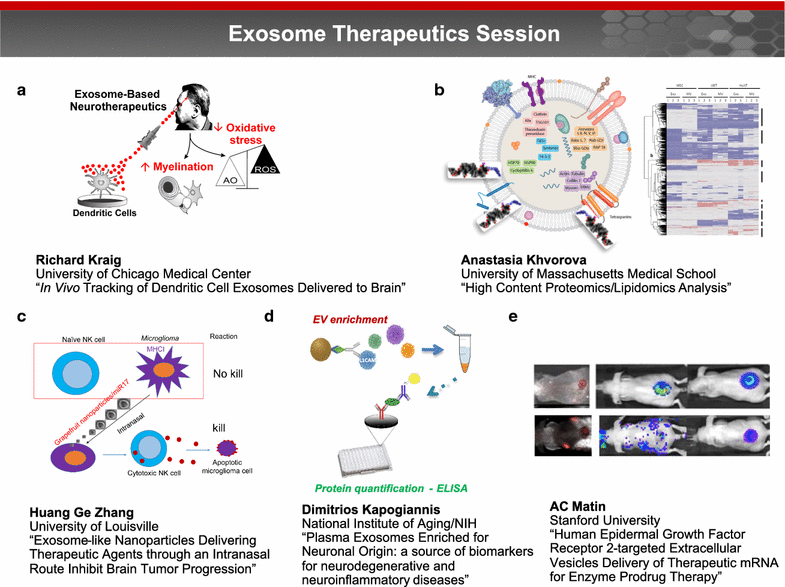
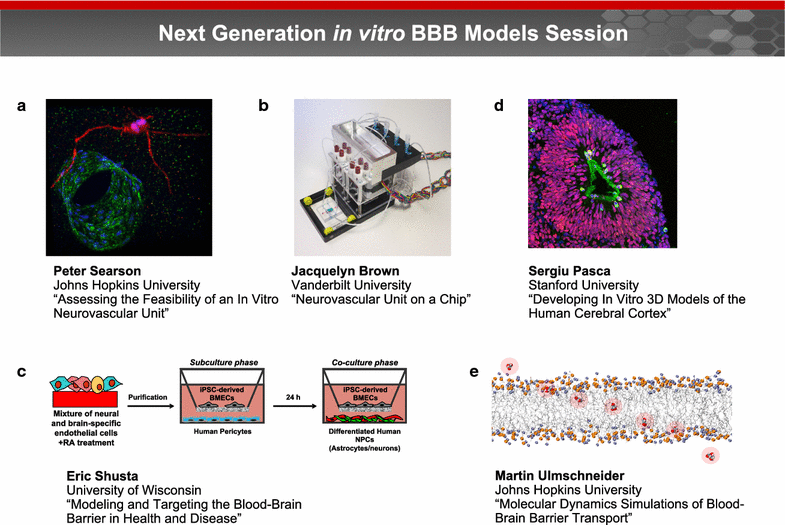
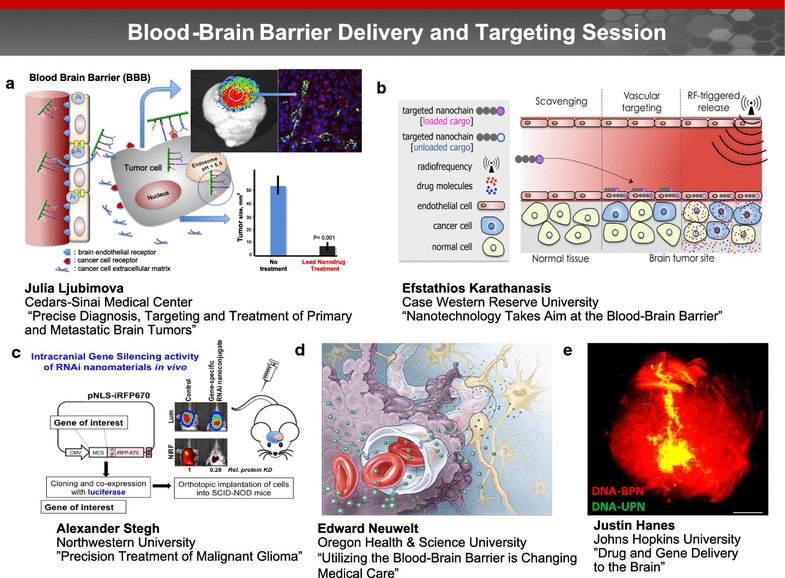
A trans-agency workshop on the blood-brain interface (BBI), sponsored by the National Heart, Lung and Blood Institute, the National Cancer Institute and the Combat Casualty Care Research Program at the Department of Defense, was conducted in Bethesda MD on June 7-8, 2016. The workshop was structured into four sessions: (1) blood sciences; (2) exosome therapeutics; (3) next generation in vitro blood-brain barrier (BBB) models; and (4) BBB delivery and targeting. The first day of the workshop focused on the physiology of the blood and neuro-vascular unit, blood or biofluid-based molecular markers, extracellular vesicles associated with brain injury, and how these entities can be employed to better evaluate injury states and/or deliver therapeutics. The second day of the workshop focused on technical advances in in vitro models, BBB manipulations and nanoparticle-based drug carrier designs, with the goal of improving drug delivery to the central nervous system. The presentations and discussions underscored the role of the BBI in brain injury, as well as the role of the BBB as both a limiting factor and a potential conduit for drug delivery to the brain. At the conclusion of the meeting, the participants discussed challenges and opportunities confronting BBI translational researchers. In particular, the participants recommended using BBI translational research to stimulate advances in diagnostics, as well as targeted delivery approaches for detection and therapy of both brain injury and disease.
Keywords: Blood–brain barrier; Cancer; Delivery; Exosomes; Extracellular vesicles; Neurodegeneration; Therapeutics; Traumatic brain injury.
Figures
Similar articles
-
From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery.Nat Rev Drug Discov. 2016 Apr;15(4):275-92. doi: 10.1038/nrd.2015.21. Epub 2016 Jan 22. Nat Rev Drug Discov. 2016. PMID: 26794270 Review.
-
New frontiers in translational research in neuro-oncology and the blood-brain barrier: report of the tenth annual Blood-Brain Barrier Disruption Consortium Meeting.Clin Cancer Res. 2005 Jan 15;11(2 Pt 1):421-8. Clin Cancer Res. 2005. PMID: 15701824
-
Overcoming the Blood-Brain Barrier: The Role of Nanomaterials in Treating Neurological Diseases.Adv Mater. 2018 Nov;30(46):e1801362. doi: 10.1002/adma.201801362. Epub 2018 Jul 31. Adv Mater. 2018. PMID: 30066406 Review.
-
Emerging Technologies for Delivery of Biotherapeutics and Gene Therapy Across the Blood-Brain Barrier.BioDrugs. 2018 Dec;32(6):547-559. doi: 10.1007/s40259-018-0309-y. BioDrugs. 2018. PMID: 30306341 Free PMC article.
-
NIH workshop on clinical translation of molecular imaging probes and technology--meeting report.Mol Imaging Biol. 2014 Oct;16(5):595-604. doi: 10.1007/s11307-014-0746-z. Mol Imaging Biol. 2014. PMID: 24833042 Free PMC article.
Cited by
-
MicroRNA-based engineering of mesenchymal stem cell extracellular vesicles for treatment of retinal ischemic disorders: Engineered extracellular vesiclesand retinal ischemia.Acta Biomater. 2023 Mar 1;158:782-797. doi: 10.1016/j.actbio.2023.01.014. Epub 2023 Jan 11. Acta Biomater. 2023. PMID: 36638942 Free PMC article.
-
Comprehensive evaluation of blood-brain barrier-forming micro-vasculatures: Reference and marker genes with cellular composition.PLoS One. 2018 May 15;13(5):e0197379. doi: 10.1371/journal.pone.0197379. eCollection 2018. PLoS One. 2018. PMID: 29763456 Free PMC article.
-
Novel window for cancer nanotheranostics: non-invasive ocular assessments of tumor growth and nanotherapeutic treatment efficacy in vivo.Biomed Opt Express. 2018 Dec 11;10(1):151-166. doi: 10.1364/BOE.10.000151. eCollection 2019 Jan 1. Biomed Opt Express. 2018. PMID: 30775090 Free PMC article.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles and Their Therapeutic Use in Central Nervous System Demyelinating Disorders.Int J Mol Sci. 2022 Mar 30;23(7):3829. doi: 10.3390/ijms23073829. Int J Mol Sci. 2022. PMID: 35409188 Free PMC article. Review.
-
The Pro-Apoptotic Effect of Silica Nanoparticles Depends on Their Size and Dose, as Well as the Type of Glioblastoma Cells.Int J Mol Sci. 2021 Mar 30;22(7):3564. doi: 10.3390/ijms22073564. Int J Mol Sci. 2021. PMID: 33808150 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
- R01 AG046619/AG/NIA NIH HHS/United States
- R01 AG039452/AG/NIA NIH HHS/United States
- R01 NS034467/NS/NINDS NIH HHS/United States
- R01 NS100459/NS/NINDS NIH HHS/United States
- UH3 TR000918/TR/NCATS NIH HHS/United States
- P01 AG052350/AG/NIA NIH HHS/United States
- RF1 AG039452/AG/NIA NIH HHS/United States
- R01 NS090904/NS/NINDS NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- R01 NS019108/NS/NINDS NIH HHS/United States
- R21 NS093368/NS/NINDS NIH HHS/United States
- R03 AG050184/AG/NIA NIH HHS/United States
- R01 AG023084/AG/NIA NIH HHS/United States
- R01 CA206220/CA/NCI NIH HHS/United States
- U01 CA151815/CA/NCI NIH HHS/United States
- R03 AG046216/AG/NIA NIH HHS/United States
- R01 CA188743/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

