Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses
- PMID: 28457665
- PMCID: PMC5475249
- DOI: 10.1016/j.ymthe.2017.03.035
Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses
Erratum in
-
Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses.Mol Ther. 2022 Aug 3;30(8):2874. doi: 10.1016/j.ymthe.2022.07.013. Epub 2022 Aug 2. Mol Ther. 2022. PMID: 35921837 Free PMC article. No abstract available.
Abstract
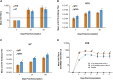
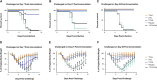
Recently, the World Health Organization confirmed 120 new human cases of avian H7N9 influenza in China resulting in 37 deaths, highlighting the concern for a potential pandemic and the need for an effective, safe, and high-speed vaccine production platform. Production speed and scale of mRNA-based vaccines make them ideally suited to impede potential pandemic threats. Here we show that lipid nanoparticle (LNP)-formulated, modified mRNA vaccines, encoding hemagglutinin (HA) proteins of H10N8 (A/Jiangxi-Donghu/346/2013) or H7N9 (A/Anhui/1/2013), generated rapid and robust immune responses in mice, ferrets, and nonhuman primates, as measured by hemagglutination inhibition (HAI) and microneutralization (MN) assays. A single dose of H7N9 mRNA protected mice from a lethal challenge and reduced lung viral titers in ferrets. Interim results from a first-in-human, escalating-dose, phase 1 H10N8 study show very high seroconversion rates, demonstrating robust prophylactic immunity in humans. Adverse events (AEs) were mild or moderate with only a few severe and no serious events. These data show that LNP-formulated, modified mRNA vaccines can induce protective immunogenicity with acceptable tolerability profiles.
Keywords: H10N8; H7N9; immunogenicity; influenza; mRNA; mRNA vaccines; pandemic; vaccines.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

