Quantitative Evaluation of Biologic Therapy Options for Psoriasis: A Systematic Review and Network Meta-Analysis
- PMID: 28457908
- PMCID: PMC5519491
- DOI: 10.1016/j.jid.2017.04.009
Quantitative Evaluation of Biologic Therapy Options for Psoriasis: A Systematic Review and Network Meta-Analysis
Erratum in
-
Corrigenda.J Invest Dermatol. 2021 Feb;141(2):463. doi: 10.1016/j.jid.2020.12.010. J Invest Dermatol. 2021. PMID: 33504440 Free PMC article. No abstract available.
Abstract
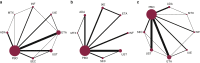
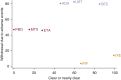
Multiple biologic treatments are licensed for psoriasis. The lack of head-to-head randomized controlled trials makes choosing between them difficult for patients, clinicians, and guideline developers. To establish their relative efficacy and tolerability, we searched MEDLINE, PubMed, Embase, and Cochrane for randomized controlled trials of licensed biologic treatments for skin psoriasis. We performed a network meta-analysis to identify direct and indirect evidence comparing biologics with one another, methotrexate, or placebo. We combined this with hierarchical cluster analysis to consider multiple outcomes related to efficacy and tolerability in combination for each treatment. Study quality, heterogeneity, and inconsistency were evaluated. Direct comparisons from 41 randomized controlled trials (20,561 participants) were included. All included biologics were efficacious compared with placebo or methotrexate at 3-4 months. Overall, cluster analysis showed adalimumab, secukinumab, and ustekinumab were comparable in terms of high efficacy and tolerability. Ixekizumab and infliximab were differentiated by very high efficacy but poorer tolerability. The lack of longer term controlled data limited our analysis to short-term outcomes. Trial performance may not equate to real-world performance, and so results need to be considered alongside real-world, long-term safety and effectiveness data. These data suggest that it is possible to discriminate between biologics to inform clinical practice and decision making (PROSPERO 2015:CRD42015017538).
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Comment on "Quantitative Evaluation of Biologic Therapy Options for Psoriasis: A Systematic Review and Network Meta-Analysis".J Invest Dermatol. 2017 Dec;137(12):2642-2644. doi: 10.1016/j.jid.2017.06.031. Epub 2017 Aug 2. J Invest Dermatol. 2017. PMID: 28780085 No abstract available.
-
Re: Quantitative Evaluation of Biologic Therapy Options for Psoriasis: A Systematic Review and Network Meta-Analysis.J Invest Dermatol. 2017 Dec;137(12):2644-2646. doi: 10.1016/j.jid.2017.07.848. Epub 2017 Aug 31. J Invest Dermatol. 2017. PMID: 28864078 No abstract available.
-
Network meta-analyses of systemic treatments for psoriasis: a critical appraisal: Original Articles: Jabbar-Lopez ZK, Yiu ZZN, Ward V et al. Quantitative evaluation of biologic therapy options for psoriasis: a systematic review and network meta-analysis. J Invest Dermatol 2017; 137:1646-54. Sbidian E, Chaimani A, Garcia-Doval I et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev 2017; 12:CD011535.Br J Dermatol. 2019 Feb;180(2):282-288. doi: 10.1111/bjd.17335. Epub 2018 Dec 21. Br J Dermatol. 2019. PMID: 30347448
-
Quantitative Evaluation of Biologic Therapy Options for Psoriasis: A Systematic Review and Network Meta-Analysis-Correction.J Invest Dermatol. 2021 Jan;141(1):177-181. doi: 10.1016/j.jid.2020.02.048. J Invest Dermatol. 2021. PMID: 33342507 No abstract available.
References
-
- Bansback N., Sizto S., Sun H.Y., Feldman S., Willian M.K., Anis A. Efficacy of systemic treatments for moderate to severe plaque psoriasis: systematic review and meta-analysis. Dermatology. 2009;219:209–218. - PubMed
-
- Barker J., Hoffmann M., Wozel G., Ortonne J.P., Zheng H., van Hoogstraten H. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1) Br J Dermatol. 2011;165:1109–1117. - PubMed
-
- Basra M.K., Salek M.S., Camilleri L., Sturkey R., Finlay A.Y. Determining the minimal clinically important difference and responsiveness of the dermatology life quality index (DLQI): further data. Dermatology. 2015;230:27–33. - PubMed
-
- Bucher H.C., Guyatt G.H., Griffith L.E., Walter S.D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

