Non-nuclear estrogen receptor alpha activation in endothelium reduces cardiac ischemia-reperfusion injury in mice
- PMID: 28457941
- PMCID: PMC5514412
- DOI: 10.1016/j.yjmcc.2017.04.004
Non-nuclear estrogen receptor alpha activation in endothelium reduces cardiac ischemia-reperfusion injury in mice
Abstract
Steroid hormone receptors including estrogen receptors (ER) classically function as ligand-regulated transcription factors. However, estrogens also elicit cellular effects through binding to extra-nuclear ER (ERα, ERβ, and G protein-coupled ER or GPER) that are coupled to kinases. How extra-nuclear ER actions impact cardiac ischemia-reperfusion (I/R) injury is unknown. We treated ovariectomized wild-type female mice with estradiol or an estrogen-dendrimer conjugate (EDC), which selectively activates extra-nuclear ER, or vehicle interventions for two weeks. I/R injury was then evaluated in isolated Langendorff perfused hearts. Two weeks of treatment with estradiol significantly decreased infarct size and improved post-ischemic contractile function. Similarly, EDC treatment significantly decreased infarct size and increased post-ischemic functional recovery compared to vehicle-treated hearts. EDC also caused an increase in myocardial protein S-nitrosylation, consistent with previous studies showing a role for this post-translational modification in cardioprotection. In further support of a role for S-nitrosylation, inhibition of nitric oxide synthase, but not soluble guanylyl cyclase blocked the EDC mediated protection. The administration of ICI182,780, which is an agonist of G-protein coupled estrogen receptor (GPER) and an antagonist of ERα and ERβ, did not result in protection; however, ICI182,780 significantly blocked EDC-mediated cardioprotection, indicating participation of ERα and/or ERβ. In studies determining the specific ER subtype and cellular target involved, EDC decreased infarct size and improved functional recovery in mice lacking ERα in cardiomyocytes. In contrast, protection was lost in mice deficient in endothelial cell ERα. Thus, extra-nuclear ERα activation in endothelium reduces cardiac I/R injury in mice, and this likely entails increased protein S-nitrosylation. Since EDC does not stimulate uterine growth, in the clinical setting EDC-like compounds may provide myocardial protection without undesired uterotrophic and cancer-promoting effects.
Keywords: Cardiomyocyte; Endothelium; Estrogen receptor; Nitric oxide signaling.
Published by Elsevier Ltd.
Conflict of interest statement
Figures

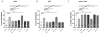


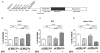

References
-
- Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–7. - PubMed
-
- Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. Jama. 1991;265:1861–7. - PubMed
-
- Hodis HN, Mack WJ, Azen SP, Lobo RA, Shoupe D, Mahrer PR, et al. Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. The New England journal of medicine. 2003;349:535–45. - PubMed
-
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) Jama. 2002;288:49–57. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

