Lysine desuccinylase SIRT5 binds to cardiolipin and regulates the electron transport chain
- PMID: 28458255
- PMCID: PMC5473227
- DOI: 10.1074/jbc.M117.785022
Lysine desuccinylase SIRT5 binds to cardiolipin and regulates the electron transport chain
Abstract
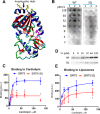
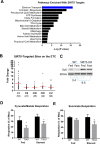
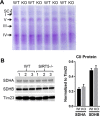
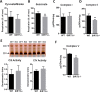
SIRT5 is a lysine desuccinylase known to regulate mitochondrial fatty acid oxidation and the urea cycle. Here, SIRT5 was observed to bind to cardiolipin via an amphipathic helix on its N terminus. In vitro, succinyl-CoA was used to succinylate liver mitochondrial membrane proteins. SIRT5 largely reversed the succinyl-CoA-driven lysine succinylation. Quantitative mass spectrometry of SIRT5-treated membrane proteins pointed to the electron transport chain, particularly Complex I, as being highly targeted for desuccinylation by SIRT5. Correspondingly, SIRT5-/- HEK293 cells showed defects in both Complex I- and Complex II-driven respiration. In mouse liver, SIRT5 expression was observed to localize strictly to the periportal hepatocytes. However, homogenates prepared from whole SIRT5-/- liver did show reduced Complex II-driven respiration. The enzymatic activities of Complex II and ATP synthase were also significantly reduced. Three-dimensional modeling of Complex II suggested that several SIRT5-targeted lysine residues lie at the protein-lipid interface of succinate dehydrogenase subunit B. We postulate that succinylation at these sites may disrupt Complex II subunit-subunit interactions and electron transfer. Lastly, SIRT5-/- mice, like humans with Complex II deficiency, were found to have mild lactic acidosis. Our findings suggest that SIRT5 is targeted to protein complexes on the inner mitochondrial membrane via affinity for cardiolipin to promote respiratory chain function.
Keywords: cardiolipin; electron transport system (ETS); mitochondria; protein acylation; sirtuin.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Fould B., Garlatti V., Neumann E., Fenel D., Gaboriaud C., and Arlaud G. J. (2010) Structural and functional characterization of the recombinant human mitochondrial trifunctional protein. Biochemistry 49, 8608–8617 - PubMed
-
- Kashfi K., Mynatt R. L., Park E. A., and Cook G. A. (2011) Membrane microenvironment regulation of carnitine palmitoyltranferases I and II. Biochem. Soc. Trans. 39, 833–837 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

