Progress on lead-free metal halide perovskites for photovoltaic applications: a review
- PMID: 28458399
- PMCID: PMC5387038
- DOI: 10.1007/s00706-017-1933-9
Progress on lead-free metal halide perovskites for photovoltaic applications: a review
Abstract
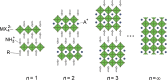
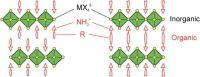
Abstract: Metal halide perovskites have revolutionized the field of solution-processable photovoltaics. Within just a few years, the power conversion efficiencies of perovskite-based solar cells have been improved significantly to over 20%, which makes them now already comparably efficient to silicon-based photovoltaics. This breakthrough in solution-based photovoltaics, however, has the drawback that these high efficiencies can only be obtained with lead-based perovskites and this will arguably be a substantial hurdle for various applications of perovskite-based photovoltaics and their acceptance in society, even though the amounts of lead in the solar cells are low. This fact opened up a new research field on lead-free metal halide perovskites, which is currently remarkably vivid. We took this as incentive to review this emerging research field and discuss possible alternative elements to replace lead in metal halide perovskites and the properties of the corresponding perovskite materials based on recent theoretical and experimental studies. Up to now, tin-based perovskites turned out to be most promising in terms of power conversion efficiency; however, also the toxicity of these tin-based perovskites is argued. In the focus of the research community are other elements as well including germanium, copper, antimony, or bismuth, and the corresponding perovskite compounds are already showing promising properties.
Keywords: Hybrid organic–inorganic materials; Material science; Semiconductor; Solar cell; Transition metals compounds.
Figures















References
-
- Green MA, Emery K, Hishikawa Y, Warta W, Dunlop ED, Levi DH, Ho-Baillie AWY. Prog Photovolt Res Appl. 2017;25:3. doi: 10.1002/pip.2855. - DOI
-
- National Renewable Energy Laboratory (NREL). http://www.nrel.gov/pv/assets/images/efficiency_chart.jpg. Accessed 12 Aug 2016
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
