Cost-effectiveness analysis of abobotulinumtoxinA for the treatment of cervical dystonia in the United Kingdom
- PMID: 28458568
- PMCID: PMC5402907
- DOI: 10.2147/CEOR.S112254
Cost-effectiveness analysis of abobotulinumtoxinA for the treatment of cervical dystonia in the United Kingdom
Abstract
Background: Cervical dystonia (CD) involves painful involuntary contraction of the neck and shoulder muscles and abnormal posture in middle-aged adults. Botulinum neurotoxin type A (BoNT-A) is effective in treating CD but little is known about its associated cost-effectiveness.
Objective: To evaluate the cost-effectiveness of abobotulinumtoxinA for treating CD from the UK payer perspective.
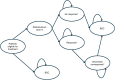
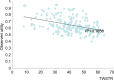
Methods: A Markov model was developed to evaluate the cost-effectiveness of abobotulinum-toxinA versus best supportive care (BSC) in CD, with a lifetime horizon and health states for response, nonresponse, secondary nonresponse, and BSC in patients with CD (mean age: 53 years; 37% male). Clinical improvement measured using Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) was mapped to utility using data from a randomized trial of abobotulinumtoxinA. Health care resource use, costs, and other inputs were from the British National Formulary, Personal Social Services Research Unit, published literature, or expert opinion. Costs and outcomes were discounted at 3.5% per annum.
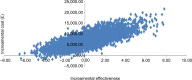
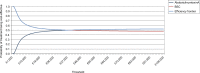
Results: In the base case, the incremental lifetime quality-adjusted life-years (QALYs) gained from abobotulinumtoxinA arm versus BSC was 0.253 per patient, whereas the incremental cost was £7,160, leading to an incremental cost-effectiveness ratio (ICER) of £30,468 per QALY. One-way sensitivity analyses showed that these results were sensitive to the proportion of responders to abobotulinumtoxinA at first injection, duration between injections, the number of reinjections allowed among primary nonresponders, and any difference in baseline TWSTRS value between the BSC and abobotulinumtoxinA arms. Probabilistic sensitivity analysis showed that abobotulinumtoxinA was cost-effective 46% and 49% of times at thresholds of £20,000 and £30,000 per QALY, respectively. Scenarios are considered including vial-sharing, productivity losses, secondary response/nonresponse at subsequent injections, 5-year time horizon, and alternative reinjection intervals for BoNT-As produced ICERs ranging from cost-saving to £40,777 per QALY, versus BSC.
Conclusion: AbobotulinumtoxinA was found to be cost-effective in treating adults with CD, at acceptable willingness-to-pay thresholds in the UK.
Keywords: abobotulinumtoxinA; botulinum neurotoxin type A; cervical dystonia; cost-effectiveness analysis.
Conflict of interest statement
Disclosure MM, SA, and KD are employees of Evidera Inc., which received consultancy fees to conduct the research from Ipsen Pharma. JD and SG are both full-time employees of Ipsen Pharma. TH has received consultancy fees from Ipsen Pharma for work relating to Spasticity management TH has also received honoraria for lectures delivered from Merz and Allergan. Ipsen Pharma did not have any influence on the interpretation of data as well as the final conclusions drawn. The authors report no other conflicts of interest in this work.
Figures
References
-
- Truong D, Brodsky M, Lew M, et al. Global dysport cervical dystonia study group Long-term efficacy and safety of botulinum toxin type A (Dysport) in cervical dystonia. Parkinsonism Relat Disord. 2010;16(5):316–323. - PubMed
-
- Electronic medicines compendium (eMC) Dysport® 300 units, Dys-port® 500 units, summary of product characteristics. [Accessed January 2016]. Available from: https://www.medicines.org.uk/emc/medicine/870.
-
- Kessler KR, Skutta M, Benecke R. Long-term treatment of cervical dystonia with botulinum toxin A: efficacy, safety, and antibody frequency. German Dystonia study group. J Neurol. 1999;246(4):265–274. - PubMed
-
- Electronic Medicines Compendium (eMC) Bortox® 100 units, Bortox® 50 units, Summary of Product Characteristics. [Accessed January 2016]. Available from: https://www.medicines.org.uk/emc/medicine/112; https://www.medicines.org.uk/emc/medicine/22562; https://www.medicines.org.uk/emc/medicine/20564.
-
- Electronic medicines compendium (eMC) Xeomin® 100 units, Xeo-min® 50 units, summary of product characteristics. [Accessed January 2016]. Available from: https://www.medicines.org.uk/emc/medicine/20666; https://www.medicines.org.uk/emc/medicine/24582.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

