Targeted Peripheral Nerve-directed Onabotulinumtoxin A Injection for Effective Long-term Therapy for Migraine Headache
- PMID: 28458982
- PMCID: PMC5404453
- DOI: 10.1097/GOX.0000000000001270
Targeted Peripheral Nerve-directed Onabotulinumtoxin A Injection for Effective Long-term Therapy for Migraine Headache
Abstract
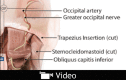
Background: Onabotulinumtoxin A (BOTOX) is an FDA-approved treatment for chronic migraine headaches (MHs) that involves on-label, high-dose administration across 31 anatomic sites. Anatomically specific peripheral nerve trigger sites have been identified that contribute to MH pathogenesis and are amenable to both BOTOX injection and surgical decompression. These sites do not always correlate with the on-label FDA-approved injection pattern, but represent a more targeted approach. The efficacy of peripheral nerve-directed BOTOX injection as an independent long-term therapeutic option has not been investigated.
Methods: The technique for peripheral nerve-directed therapeutic long-term BOTOX injection is described. A retrospective review was subsequently completed for 223 patients with MH. Sixty-six patients elected to proceed with diagnostic BOTOX injections. Of these, 24 continued long-term therapeutic BOTOX injections, whereas 42 matriculated to surgery. Outcomes were tracked.
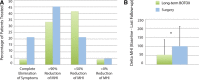
Results: Initial outcomes included significant improvement in migraine headache index (MHI) (53.5 ± 83.0, P < 0.006), headache days/mo (9.2 ± 12.7, P < 0.0009), and migraine severity (2.6 ± 2.5, P < 0.00008) versus baseline. MHI improved from the initiation of diagnostic injections to the establishment of steady-state injections (P < 0.002), and further improved over time (P < 0.05, mean follow-up 615 days) with no desensitization observed. Decompressive surgery resulted in significant improvement in MHI (100.8 ± 109.7, P < 0.0000005), headache days/mo (10.8 ± 12.7, P < 0.000002), migraine severity (3.0 ± 3.8, P < 0.00001), and migraine duration in hours (16.8 ± 21.6, P < 0.0007). MHI improvement with surgery was better than long-term BOTOX injections (P < 0.05).
Conclusions: Though inferior to surgical decompression, preliminary data demonstrate that targeted peripheral nerve-directed BOTOX injection is an effective primary therapy for MH representing a possible alternative to nondirected BOTOX injection with decreased dosage requirements and potentially decreased cost.
Figures




References
-
- Stewart WF, Simon D, Shechter A, et al. Population variation in migraine prevalence: a meta-analysis. J Clin Epidemiol. 1995;48:269–280.. - PubMed
-
- Stewart WF, Wood C, Reed ML, et al. ; AMPP Advisory Group. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170–1178.. - PubMed
-
- BOTOX Prescribing Information. Revised 8/2015. http://www.allergan.com/assets/pdf/botox_cosmetic_pi.pdf. Accessed February 27, 2017.
-
- Aurora SK, Dodick DW, Turkel CC, et al. ; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793–803.. - PubMed
-
- Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358–1373.. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
