Re-Engineering Alzheimer Clinical Trials: Global Alzheimer's Platform Network
- PMID: 28459045
- PMCID: PMC5408881
- DOI: 10.14283/jpad.2016.93
Re-Engineering Alzheimer Clinical Trials: Global Alzheimer's Platform Network
Abstract
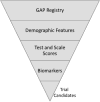
Alzheimer's disease (AD) drug development is costly, time-consuming, and inefficient. Trial site functions, trial design, and patient recruitment for trials all require improvement. The Global Alzheimer Platform (GAP) was initiated in response to these challenges. Four GAP work streams evolved in the US to address different trial challenges: 1) registry-to-cohort web-based recruitment; 2) clinical trial site activation and site network construction (GAP-NET); 3) adaptive proof-of-concept clinical trial design; and 4) finance and fund raising. GAP-NET proposes to establish a standardized network of continuously funded trial sites that are highly qualified to perform trials (with established clinical, biomarker, imaging capability; certified raters; sophisticated management system. GAP-NET will conduct trials for academic and biopharma industry partners using standardized instrument versions and administration. Collaboration with the Innovative Medicines Initiative (IMI) European Prevention of Alzheimer's Disease (EPAD) program, the Canadian Consortium on Neurodegeneration in Aging (CCNA) and other similar international initiatives will allow conduct of global trials. GAP-NET aims to increase trial efficiency and quality, decrease trial redundancy, accelerate cohort development and trial recruitment, and decrease trial costs. The value proposition for sites includes stable funding and uniform training and trial execution; the value to trial sponsors is decreased trial costs, reduced time to execute trials, and enhanced data quality. The value for patients and society is the more rapid availability of new treatments for AD.
Keywords: Alzheimer’s disease; Global Alzheimer Platform; certification; clinical trials; drug development; drug discovery; recruitment; registry.
Figures
References
-
- Alzheimer’s Disease International. Prince M, Guerchet M, Prina M. Policy Brief for Heads of Government: The Global Impact of Dementia 2013–2050. London: Alzheimer’s Disease International; 2013.
-
- The New York Academy of Sciences. Alzheimers Disease Summit: The Path to 2025 Summary and Strategy Report. New York, NY: The New York Academy of Sciences; 2013. Dec 9, The Global CEO Initiative on Alzheimer’s Disease.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous