In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis
- PMID: 28459449
- PMCID: PMC5462879
- DOI: 10.1038/nbt.3836
In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis
Erratum in
-
Corrigendum: In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis.Nat Biotechnol. 2017 Dec 8;35(12):1211. doi: 10.1038/nbt1217-1211a. Nat Biotechnol. 2017. PMID: 29220013
Abstract
In vivo interrogation of the function of genes implicated in tumorigenesis is limited by the need to generate and cross germline mutant mice. Here we describe approaches to model colorectal cancer (CRC) and metastasis, which rely on in situ gene editing and orthotopic organoid transplantation in mice without cancer-predisposing mutations. Autochthonous tumor formation is induced by CRISPR-Cas9-based editing of the Apc and Trp53 tumor suppressor genes in colon epithelial cells and by orthotopic transplantation of Apc-edited colon organoids. ApcΔ/Δ;KrasG12D/+;Trp53Δ/Δ (AKP) mouse colon organoids and human CRC organoids engraft in the distal colon and metastasize to the liver. Finally, we apply the orthotopic transplantation model to characterize the clonal dynamics of Lgr5+ stem cells and demonstrate sequential activation of an oncogene in established colon adenomas. These experimental systems enable rapid in vivo characterization of cancer-associated genes and reproduce the entire spectrum of tumor progression and metastasis.
Conflict of interest statement
Figures
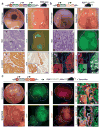

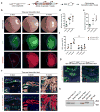
Comment in
-
Cancer models: Tailored mouse models.Nat Rev Cancer. 2017 Jul;17(7):395. doi: 10.1038/nrc.2017.45. Epub 2017 Jun 9. Nat Rev Cancer. 2017. PMID: 28642600 No abstract available.
References
-
- Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. - PubMed
-
- Su LK, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. - PubMed
-
- Roper J, Hung KE. Priceless GEMMs: genetically engineered mouse models for colorectal cancer drug development. Trends Pharmacol Sci. 2012;33:449–455. - PubMed
Publication types
MeSH terms
Grants and funding
- P30 DK043351/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- R01 CA017007/CA/NCI NIH HHS/United States
- K08 CA198002/CA/NCI NIH HHS/United States
- R00 AG045144/AG/NIA NIH HHS/United States
- R01 CA120198/CA/NCI NIH HHS/United States
- R01 AI050631/AI/NIAID NIH HHS/United States
- R01 AI040146/AI/NIAID NIH HHS/United States
- P30 CA147882/CA/NCI NIH HHS/United States
- R01 CA184956/CA/NCI NIH HHS/United States
- K99 CA187317/CA/NCI NIH HHS/United States
- K99 AG045144/AG/NIA NIH HHS/United States
- R01 CA034992/CA/NCI NIH HHS/United States
- R01 CA211184/CA/NCI NIH HHS/United States
- C06 CA062520/CA/NCI NIH HHS/United States
- U54 CA163109/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

