Addressable Direct-Write Nanoscale Filament Formation and Dissolution by Nanoparticle-Mediated Bipolar Electrochemistry
- PMID: 28459548
- PMCID: PMC8276755
- DOI: 10.1021/acsnano.7b01657
Addressable Direct-Write Nanoscale Filament Formation and Dissolution by Nanoparticle-Mediated Bipolar Electrochemistry
Abstract
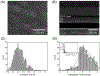
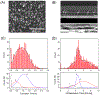
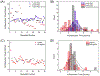
Nanoscale conductive filaments, usually associated with resistive memory or memristor technology, may also be used for chemical sensing and nanophotonic applications; however, realistic implementation of the technology requires precise knowledge of the conditions that control the formation and dissolution of filaments. Here we describe and characterize an addressable direct-write nanoelectrochemical approach to achieve repeatable formation/dissolution of Ag filaments across a ∼100 nm poly(ethylene oxide) (PEO) film containing either Ag+ alone or Ag+ together with 50 nm Ag-nanoparticles acting as bipolar electrodes. Using a conductive AFM tip, formation occurs when the PEO film is subjected to a forward bias, and dissolution occurs under reverse bias. Formation-dissolution kinetics were studied for three film compositions: Ag|PEO-Ag+, Ag|poly(ethylene glycol) monolayer-PEO-Ag+, and Ag|poly(ethylene glycol) monolayer-PEO-Ag+/Ag-nanoparticle. Statistical analysis shows that the distribution of formation times exhibits Gaussian behavior, and the fastest average initial formation time occurs for the Ag|PEO-Ag+ system. In contrast, formation in the presence of Ag nanoparticles likely proceeds by a noncontact bipolar electrochemical mechanism, exhibiting the slowest initial filament formation. Dissolution times are log-normal for all three systems, and repeated reformation of filaments from previously formed structures is characterized by rapid regrowth. The direct-write bipolar electrochemical deposition/dissolution strategy developed here presents an approach to reconfigurable, noncontact in situ wiring of nanoparticle arrays-thereby enabling applications where actively controlled connectivity of nanoparticle arrays is used to manipulate nanoelectronic and nanophotonic behavior. The system further allows for facile manipulation of experimental conditions while simultaneously characterizing surface conditions and filament formation/dissolution kinetics.
Keywords: conductive AFM; conductive filament; formation−dissolution kinetics; resistive switching; solid polymer electrolyte.
Figures


References
-
- Yoon J; Lee J; Choi H; Park J-B; Seong D.-j.; Lee W; Cho C; Kim S; Hwang H. Analysis of Copper Ion Filaments and Retention of Dual-Layered Devices for Resistance Random Access Memory Applications Microelectron. Eng 2009, 86, 1929–1932
-
- Valov I; Waser R; Jameson JR; Kozicki MN Electrochemical Metallization Memories-Fundamentals, Applications, Prospects Nanotechnology 2011, 22, 254003. - PubMed
-
- Patolsky F; Zheng G; Lieber CM Nanowire-Based Biosensors Anal. Chem 2006, 78, 4260–4269 - PubMed
-
- Hwang T-W; Branagan SP; Bohn PW Chemical Noise Produced by Equilibrium Adsorption/Desorption of Surface Pyridine at Au–Ag–Au Bimetallic Atom-Scale Junctions Studied by Fluctuation Spectroscopy J. Am. Chem. Soc 2013, 135, 4522–4528 - PubMed
-
- Dong S; Zhang K; Yu Z; Fan JA Electrochemically Programmable Plasmonic Antennas ACS Nano 2016, 10, 6716–6724 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

