Asymmetric Synthesis of Second-Generation Light-Driven Molecular Motors
- PMID: 28459576
- PMCID: PMC5442604
- DOI: 10.1021/acs.joc.7b00852
Asymmetric Synthesis of Second-Generation Light-Driven Molecular Motors
Abstract
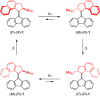
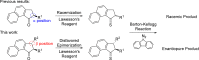
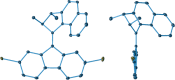
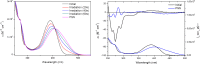
The enantiomeric homogeneity of light-driven molecular motors based on overcrowded alkenes is crucial in their application as either unidirectional rotors or as chiral multistate switches. It was challenging to obtain these compounds as single enantiomers via the established synthetic procedures due to loss of optical purity in the key step, i.e., the Barton-Kellogg olefination reaction. Searching for strategies to avoid racemization, a new class of light-driven molecular motors was designed, synthesized, and studied. The stereochemical integrity was fully preserved throughout the synthesis, and on the basis of photochemical and kinetic studies using UV/vis, CD, and 1H NMR spectroscopy, it was established that they still function properly as unidirectional molecular motors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Molecular Switches, 2nd ed; Browne W. R., Feringa B. L., Eds; Wiley-VCH: Weinheim, 2011.
- From Non-Covalent Assemblies to Molecular Machines; Sauvage J. P., Gaspard P., Eds; Wiley-VCH: Weinheim, 2010.
- Molecular Devices and Machines: Concepts and Perspectives for the Nanoworld; Balzani V., Credi A., Venturi M., Eds; Wiley-VCH: Weinheim, 2008.
- Cheng C.; Stoddart J. F. ChemPhysChem 2016, 17, 1780.10.1002/cphc.201501155. - DOI - PubMed
- Erbas-Cakmak S.; Leigh D. A.; McTernan C. T.; Nussbaumer A. L. Chem. Rev. 2015, 115, 10081.10.1021/acs.chemrev.5b00146. - DOI - PMC - PubMed
- Coskun A.; Banaszak M.; Astumian R. D.; Stoddart J. F.; Grzybowski Chem. Soc. Rev. 2012, 41, 19.10.1039/C1CS15262A. - DOI - PubMed
- Kinbara K.; Aida T. Chem. Rev. 2005, 105, 1377.10.1021/cr030071r. - DOI - PubMed
-
-
Selected examples:
- Huang T. J.; Brough B.; Ho C.-M.; Liu Y.; Flood A. H.; Bonvallet P. A.; Tseng H.-R.; Stoddart J. F.; Baller M.; Magonov S. Appl. Phys. Lett. 2004, 85, 5391.10.1063/1.1826222. - DOI
- Berná J.; Leigh D. A.; Lubomska M.; Mendoza S. M.; Pérez E. M.; Rudolf P.; Teobaldi G.; Zerbetto F. Nat. Mater. 2005, 4, 704–710. 10.1038/nmat1455. - DOI - PubMed
-
-
-
Selected examples:
- Li Q.; Fuks G.; Moulin E.; Maaloum M.; Rawiso M.; Kulic I.; Foy J. T.; Giuseppone N. Nat. Nanotechnol. 2015, 10, 161.10.1038/nnano.2014.315. - DOI - PubMed
- Foy J.; Li Q.; Goujon A.; Colard-Itté J.-R.; Fuks G.; Moulin E.; Schiffmann O.; Dattler D.; Funeriu D.; Giuseppone N. Nat. Nanotechnol. 2017, 10.1038/nnano.2017.28. - DOI - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

