Transplantation of HGF gene-engineered skeletal myoblasts improve infarction recovery in a rat myocardial ischemia model
- PMID: 28459804
- PMCID: PMC5411067
- DOI: 10.1371/journal.pone.0175807
Transplantation of HGF gene-engineered skeletal myoblasts improve infarction recovery in a rat myocardial ischemia model
Erratum in
-
Correction: Transplantation of HGF gene-engineered skeletal myoblasts improve infarction recovery in a rat myocardial ischemia model.PLoS One. 2018 Apr 19;13(4):e0196413. doi: 10.1371/journal.pone.0196413. eCollection 2018. PLoS One. 2018. PMID: 29672588 Free PMC article.
Retraction in
-
Retraction: Transplantation of HGF gene-engineered skeletal myoblasts improve infarction recovery in a rat myocardial ischemia model.PLoS One. 2023 Nov 8;18(11):e0294365. doi: 10.1371/journal.pone.0294365. eCollection 2023. PLoS One. 2023. PMID: 37939094 Free PMC article. No abstract available.
Abstract
Background: Skeletal myoblast transplantation seems a promising approach for the repair of myocardial infarction (MI). However, the low engraftment efficacy and impaired angiogenic ability limit the clinical efficiency of the myoblasts. Gene engineering with angiogenic growth factors promotes angiogenesis and enhances engraftment of transplanted skeletal myoblasts, leading to improved infarction recovery in myocardial ischemia. The present study evaluated the therapeutic effects of hepatocyte growth factor (HGF) gene-engineered skeletal myoblasts on tissue regeneration and restoration of heart function in a rat MI model.
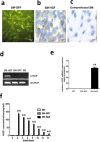
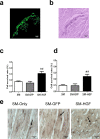
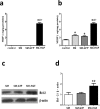
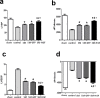
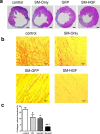
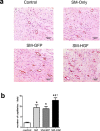
Methods and results: The skeletal myoblasts were isolated, expanded, and transduced with adenovirus carrying the HGF gene (Ad-HGF). Male SD rats underwent ligation of the left anterior descending coronary artery. After 2 weeks, the surviving rats were randomized into four groups and treated with skeletal myoblasts by direct injection into the myocardium. The survival and engraftment of skeletal myoblasts were determined by real-time PCR and in situ hybridization. The cardiac function with hemodynamic index and left ventricular architecture were monitored; The adenovirus-mediated-HGF gene transfection increases the HGF expression and promotes the proliferation of skeletal myoblasts in vitro. Transplantation of HGF-engineered skeletal myoblasts results in reduced infarct size and collagen deposition, increased vessel density, and improved cardiac function in a rat MI model. HGF gene modification also increases the myocardial levels of HGF, VEGF, and Bcl-2 and enhances the survival and engraftment of skeletal myoblasts.
Conclusions: HGF engineering improves the regenerative effect of skeletal myoblasts on MI by enhancing their survival and engraftment ability.
Conflict of interest statement
Figures


References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation, 2015. 131(4): p. e29–322. doi: 10.1161/CIR.0000000000000152 - DOI - PubMed
-
- Taylor DA, Zenovich AG. Cell therapy for left ventricular remodeling. Curr Heart Fail Rep, 2007. 4(1): p. 3–10. - PubMed
-
- Schachinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med, 2006. 355(12): p. 1210–21. doi: 10.1056/NEJMoa060186 - DOI - PubMed
-
- Henning RJ, Stem cells in cardiac repair. Future Cardiol, 2011. 7(1): p. 99–117. doi: 10.2217/fca.10.109 - DOI - PubMed
-
- Guarita-Souza LC, Carvalho KA, Woitowicz V, Rebelatto C, Senegaglia A, Hansen P, et al. Simultaneous autologous transplantation of cocultured mesenchymal stem cells and skeletal myoblasts improves ventricular function in a murine model of Chagas disease. Circulation, 2006. 114(1 Suppl): p. I120–4. doi: 10.1161/CIRCULATIONAHA.105.000646 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

