Gene expression patterns in the progression of canine copper-associated chronic hepatitis
- PMID: 28459846
- PMCID: PMC5411060
- DOI: 10.1371/journal.pone.0176826
Gene expression patterns in the progression of canine copper-associated chronic hepatitis
Abstract
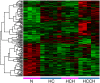
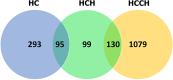
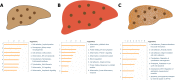
Copper is an essential trace element, but can become toxic when present in abundance. The severe effects of copper-metabolism imbalance are illustrated by the inherited disorders Wilson disease and Menkes disease. The Labrador retriever dog breed is a novel non-rodent model for copper-storage disorders carrying mutations in genes known to be involved in copper transport. Besides disease initiation and progression of copper accumulation, the molecular mechanisms and pathways involved in progression towards copper-associated chronic hepatitis still remain unclear. Using expression levels of targeted candidate genes as well as transcriptome micro-arrays in liver tissue of Labrador retrievers in different stages of copper-associated hepatitis, pathways involved in progression of the disease were studied. At the initial phase of increased hepatic copper levels, transcriptomic alterations in livers mainly revealed enrichment for cell adhesion, developmental, inflammatory, and cytoskeleton pathways. Upregulation of targeted MT1A and COMMD1 mRNA shows the liver's first response to rising intrahepatic copper concentrations. In livers with copper-associated hepatitis mainly an activation of inflammatory pathways is detected. Once the hepatitis is in the chronic stage, transcriptional differences are found in cell adhesion adaptations and cytoskeleton remodelling. In view of the high similarities in copper-associated hepatopathies between men and dog extrapolation of these dog data into human biomedicine seems feasible.
Conflict of interest statement
Figures


References
-
- de Romana DL, Olivares M, Uauy R, Araya M. Risks and benefits of copper in light of new insights of copper homeostasis. J Trace Elem Med Biol. 2011;25: 3–13. doi: 10.1016/j.jtemb.2010.11.004 - DOI - PubMed
-
- van den Berghe PV, Klomp LW. Posttranslational regulation of copper transporters. J Biol Inorg Chem. 2010;15: 37–46. doi: 10.1007/s00775-009-0592-7 - DOI - PubMed
-
- Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189: 147–163. - PubMed
-
- Freedman JH, Ciriolo MR, Peisach J. The role of glutathione in copper metabolism and toxicity. J Biol Chem. 1989;264: 5598–5605. - PubMed
-
- Valko M, Jomova K, Rhodes CJ, Kuca K, Musilek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016;90: 1–37. doi: 10.1007/s00204-015-1579-5 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

