The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis
- PMID: 28460416
- PMCID: PMC5664178
- DOI: 10.1002/jcp.25976
The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis
Abstract
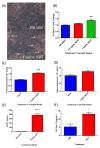
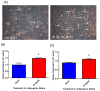
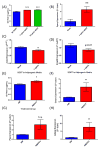
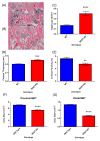
The bone marrow niche is a dynamic and complex microenvironment that can both regulate, and be regulated by the bone matrix. Within the bone marrow (BM), mesenchymal stromal cell (MSC) precursors reside in a multi-potent state and retain the capacity to differentiate down osteoblastic, adipogenic, or chondrogenic lineages in response to numerous biochemical cues. These signals can be altered in various pathological states including, but not limited to, osteoporotic-induced fracture, systemic adiposity, and the presence of bone-homing cancers. Herein we provide evidence that signals from the bone matrix (osteocytes) determine marrow adiposity by regulating adipogenesis in the bone marrow. Specifically, we found that physiologically relevant levels of Sclerostin (SOST), which is a Wnt-inhibitory molecule secreted from bone matrix-embedded osteocytes, can induce adipogenesis in 3T3-L1 cells, mouse ear- and BM-derived MSCs, and human BM-derived MSCs. We demonstrate that the mechanism of SOST induction of adipogenesis is through inhibition of Wnt signaling in pre-adipocytes. We also demonstrate that a decrease of sclerostin in vivo, via both genetic and pharmaceutical methods, significantly decreases bone marrow adipose tissue (BMAT) formation. Overall, this work demonstrates a direct role for SOST in regulating fate determination of BM-adipocyte progenitors. This provides a novel mechanism for which BMAT is governed by the local bone microenvironment, which may prove relevant in the pathogenesis of certain diseases involving marrow adipose. Importantly, with anti-sclerostin therapy at the forefront of osteoporosis treatment and a greater recognition of the role of BMAT in disease, these data are likely to have important clinical implications.
Keywords: bone marrow adipose; bone marrow microenvironment; osteocyte-derived factors; sclerostin.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
Conflicts of Interest for this work: Michaela Kneissel and Ina Kramer are employees of Novartis Pharma. The other authors have no conflicts of interest.
Figures

References
-
- Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. The Journal of biological chemistry. 2005;280(50):41342–51. - PubMed
-
- Amrein K, Amrein S, Drexler C, Dimai HP, Dobnig H, Pfeifer K, Tomaschitz A, Pieber TR, Fahrleitner-Pammer A. Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. The Journal of clinical endocrinology and metabolism. 2012;97(1):148–54. - PubMed
-
- Costa AG, Bilezikian JP, Lewiecki EM. Update on romosozumab: a humanized monoclonal antibody to sclerostin. Expert opinion on biological therapy. 2014;14(5):697–707. - PubMed
-
- Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25(9):2078–88. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

