Early outcome of early-goal directed therapy for patients with sepsis or septic shock: a systematic review and meta-analysis of randomized controlled trials
- PMID: 28460438
- PMCID: PMC5432353
- DOI: 10.18632/oncotarget.15550
Early outcome of early-goal directed therapy for patients with sepsis or septic shock: a systematic review and meta-analysis of randomized controlled trials
Abstract
Various trials and meta-analyses have reported conflicting results concerning the application of early goal-directed therapy (EGDT) for sepsis and septic shock. The aim of this study was to update the evidence by performing a systematic review and meta-analysis. Multiple databases were searched from initial through August, 2016 for randomized controlled trials (RCTs) which investigated the associations between the use of EGDT and mortality in patients with sepsis or septic shock. Meta-analysis was performed using random-effects model and heterogeneity was examined through subgroup analyses. The primary outcome of interest was patient all-cause mortality including hospital or ICU mortality. Seventeen RCTs including 6207 participants with 3234 in the EGDT group and 2973 in the control group were eligible for this study. Meta-analysis showed that EGDT did not significantly reduce hospital or intensive care unit (ICU) mortality (relative risk [RR] 0.89, 95% CI 0.78 to 1.02) compared with control group for patients with sepsis or septic shock. The findings of subgroup analyses stratified by study region, number of research center, year of enrollment, clinical setting, sample size, timing of EGDT almost remained constant with that of the primary analysis. Our findings provide evidence that EGDT offers neutral survival effects for patients with sepsis or septic shock. Further meta-analyses based on larger well-designed RCTs or individual patient data meta-analysis are required to explore the survival benefits of EDGT in patients with sepsis or septic shock.
Keywords: early-goal directed therapy; meta-analysis; randomized controlled trial; sepsis; septic shock.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures
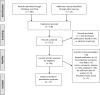

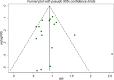
References
-
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. - PubMed
-
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. - PMC - PubMed
-
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

