Histamine therapeutic efficacy in metastatic melanoma: Role of histamine H4 receptor agonists and opportunity for combination with radiation
- PMID: 28460440
- PMCID: PMC5432273
- DOI: 10.18632/oncotarget.15594
Histamine therapeutic efficacy in metastatic melanoma: Role of histamine H4 receptor agonists and opportunity for combination with radiation
Abstract
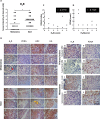
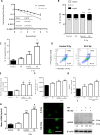
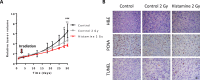
The aims of the work were to improve our knowledge of the role of H4R in melanoma proliferation and assess in vivo the therapeutic efficacy of histamine, clozapine and JNJ28610244, an H4R agonist, in a preclinical metastatic model of melanoma. Additionally, we aimed to investigate the combinatorial effect of histamine and gamma radiation on the radiobiological response of melanoma cells.Results indicate that 1205Lu metastatic melanoma cells express H4R and that histamine inhibits proliferation, in part through the stimulation of the H4R, and induces cell senescence and melanogenesis. Daily treatment with H4R agonists (1 mg/kg, sc) exhibited a significant in vivo antitumor effect and importantly, compounds reduced metastatic potential, particularly in the group treated with JNJ28610244, the H4R agonist with higher specificity. H4R is expressed in benign and malignant lesions of melanocytic lineage, highlighting the potential clinical use of histamine and H4R agonists. In addition, histamine increased radiosensitivity of melanoma cells in vitro and in vivo. We conclude that stimulation of H4R by specific ligands may represent a novel therapeutic strategy in those tumors that express this receptor. Furthermore, through increasing radiation-induced response, histamine could improve cancer radiotherapy for the treatment of melanoma.
Keywords: H4R; JNJ28610244; experimental melanoma; ionizing radiation; tumor growth.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




Similar articles
-
Role of H4 receptor in histamine-mediated responses in human melanoma.Melanoma Res. 2011 Oct;21(5):395-404. doi: 10.1097/CMR.0b013e328347ee53. Melanoma Res. 2011. PMID: 21691231
-
Study of the antitumour effects and the modulation of immune response by histamine in breast cancer.Br J Cancer. 2020 Feb;122(3):348-360. doi: 10.1038/s41416-019-0636-x. Epub 2019 Nov 21. Br J Cancer. 2020. PMID: 31748740 Free PMC article.
-
Antitumor activity of histamine and clozapine in a mouse experimental model of human melanoma.J Dermatol Sci. 2013 Dec;72(3):252-62. doi: 10.1016/j.jdermsci.2013.07.012. Epub 2013 Aug 8. J Dermatol Sci. 2013. PMID: 23999004
-
Histamine H4 receptor: insights into a potential therapeutic target in breast cancer.Front Biosci (Schol Ed). 2015 Jun 1;7(1):1-9. doi: 10.2741/S420. Front Biosci (Schol Ed). 2015. PMID: 25961682 Review.
-
A new generation of anti-histamines: Histamine H4 receptor antagonists on their way to the clinic.Curr Opin Drug Discov Devel. 2009 Sep;12(5):628-43. Curr Opin Drug Discov Devel. 2009. PMID: 19736622 Review.
Cited by
-
Immunomodulatory role of histamine H4 receptor in breast cancer.Br J Cancer. 2019 Jan;120(1):128-138. doi: 10.1038/s41416-018-0173-z. Epub 2018 Jul 10. Br J Cancer. 2019. PMID: 29988113 Free PMC article.
-
Pathophysiological Role of Histamine H4 Receptor in Cancer: Therapeutic Implications.Front Pharmacol. 2019 Jun 5;10:556. doi: 10.3389/fphar.2019.00556. eCollection 2019. Front Pharmacol. 2019. PMID: 31231212 Free PMC article.
-
Nanomicellar Formulations Loaded with Histamine and Paclitaxel as a New Strategy to Improve Chemotherapy for Breast Cancer.Int J Mol Sci. 2023 Feb 10;24(4):3546. doi: 10.3390/ijms24043546. Int J Mol Sci. 2023. PMID: 36834958 Free PMC article.
-
Canonical and Non-Canonical Antipsychotics' Dopamine-Related Mechanisms of Present and Next Generation Molecules: A Systematic Review on Translational Highlights for Treatment Response and Treatment-Resistant Schizophrenia.Int J Mol Sci. 2023 Mar 21;24(6):5945. doi: 10.3390/ijms24065945. Int J Mol Sci. 2023. PMID: 36983018 Free PMC article.
-
Histamine in cancer immunology and immunotherapy. Current status and new perspectives.Pharmacol Res Perspect. 2021 Oct;9(5):e00778. doi: 10.1002/prp2.778. Pharmacol Res Perspect. 2021. PMID: 34609067 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

