Up-regulated expression of substance P in CD8+ T cells and NK1R on monocytes of atopic dermatitis
- PMID: 28460633
- PMCID: PMC5412038
- DOI: 10.1186/s12967-017-1196-6
Up-regulated expression of substance P in CD8+ T cells and NK1R on monocytes of atopic dermatitis
Abstract
Background: Large numbers of CD8+ T cells were observed in atopic dermatitis (AD) skin, and monocytes from AD patients showed increased prostaglandin E2 production. However, little is known about the expression of substance P (SP) and its receptor NK1R in blood leukocytes of patients with AD.
Objective: To explore the expression of SP and NK1R in leukocytes of AD and the influence of allergens on SP and NK1R expression.
Methods: The expression levels of SP and NK1R in patients with AD were examined by flow cytometry, ELISA and a mouse AD model.
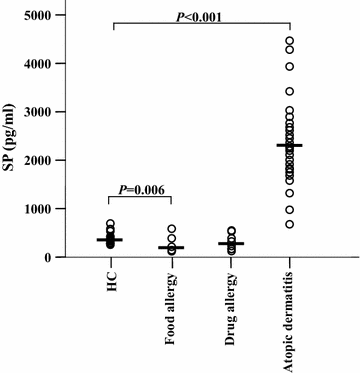
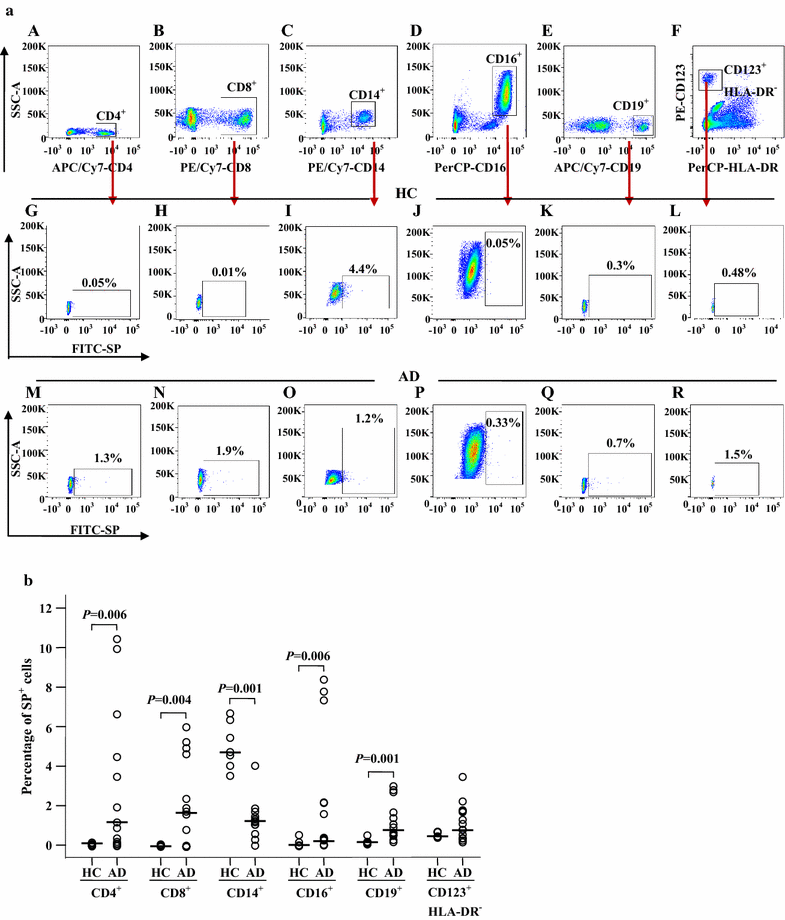
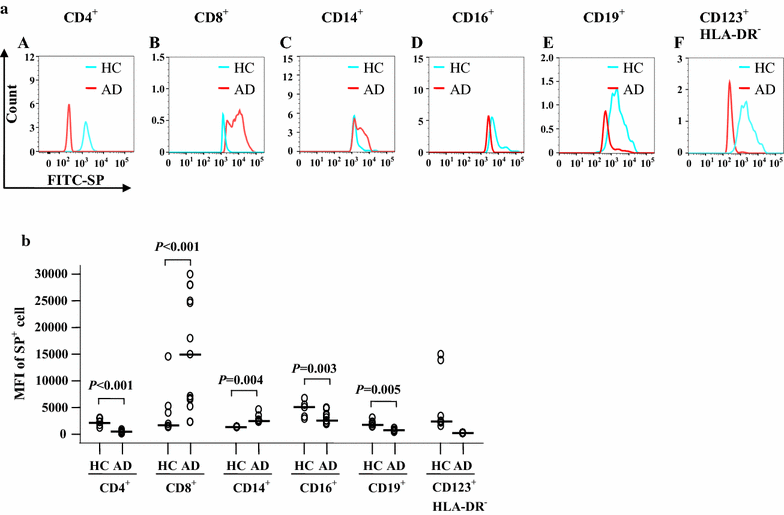
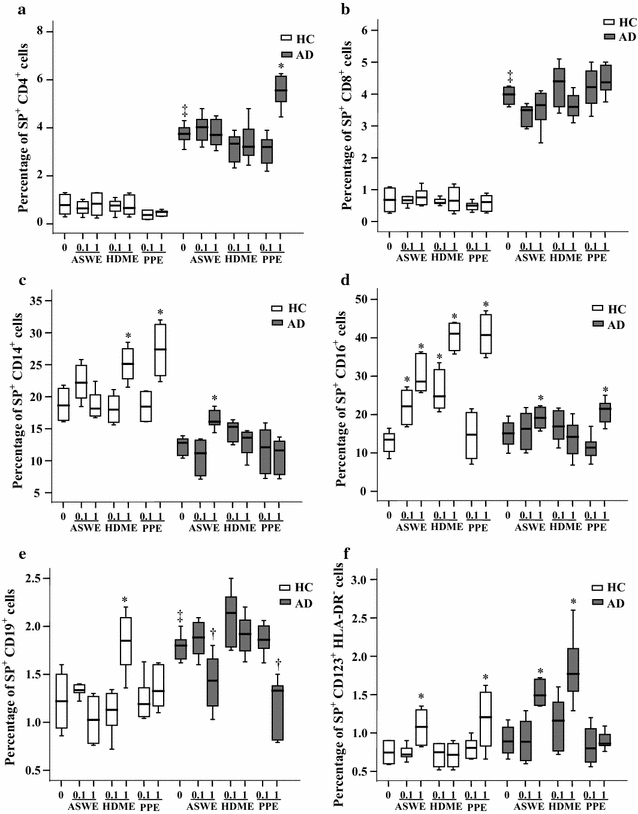
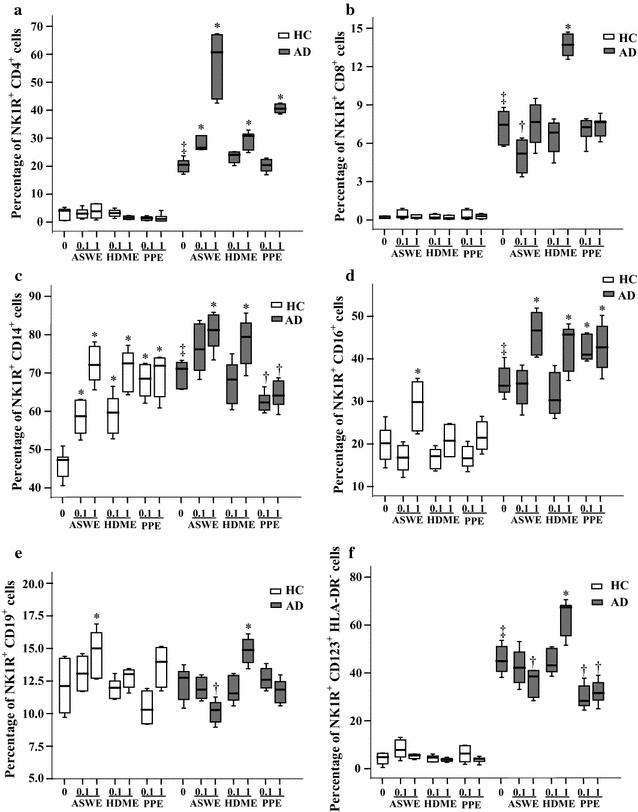
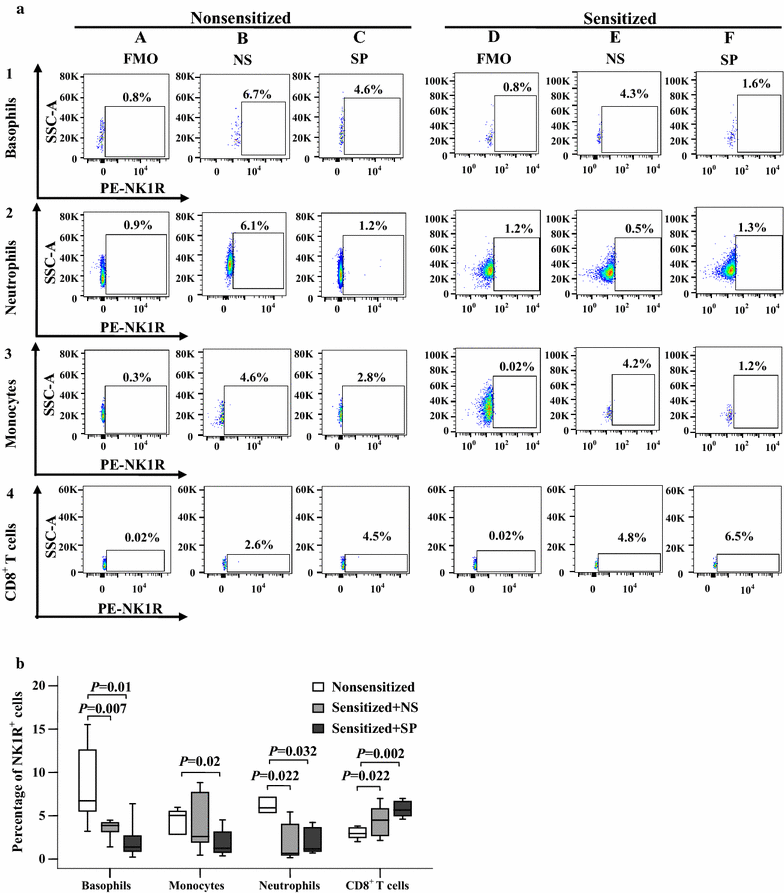
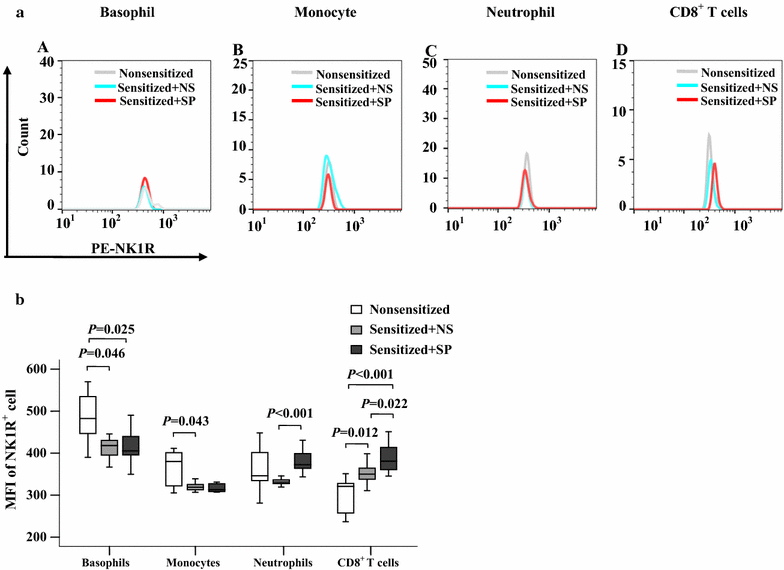
Results: The plasma SP level was 4.9-fold higher in patients with AD than in HC subjects. Both the percentage of SP expression in the population and mean fluorescence intensity (MFI) of SP expression were elevated in CD8+ T cells in the blood of AD patients. However, both the CD14+NK1R+ population and MFI of NK1R expression on CD14+ cells were enhanced in the blood of AD patients. Allergens ASWE, HDME and PPE failed to up-regulate SP expression in CD8+ T cells. However, allergens ASWE and HDME both enhanced NK1R expression on CD14+ blood leukocytes regardless of AD or HC subjects. OVA-sensitized AD mice showed an elevated proportion and MFI of SP-expressing CD8+ T cells in the blood, which agrees with the SP expression situation in human AD blood. Injection of SP into mouse skin did not up-regulate NK1R expression on monocytes.
Conclusions: An elevated plasma SP level, up-regulated expression of SP and NK1R indicate that the SP/NK1R complex is important in the development of AD. Therefore, SP and NK1R antagonist or blocker agents may help to treat patients with AD. Trial registration Registration number: ChiCTR-BOC-16010279; Registration date: Dec., 28, 2016; retrospectively registered.
Keywords: Allergen; NK1R; Substance P; T cell.
Figures




Similar articles
-
Upregulated expression of substance P and NK1R in blood monocytes and B cells of patients with allergic rhinitis and asthma.Clin Exp Immunol. 2022 Oct 21;210(1):39-52. doi: 10.1093/cei/uxac074. Clin Exp Immunol. 2022. PMID: 36001730 Free PMC article.
-
Upregulated expression of substance P (SP) and NK1R in eczema and SP-induced mast cell accumulation.Cell Biol Toxicol. 2017 Aug;33(4):389-405. doi: 10.1007/s10565-016-9379-0. Epub 2017 Feb 2. Cell Biol Toxicol. 2017. PMID: 28154998
-
Upregulated expression of substance P in basophils of the patients with chronic spontaneous urticaria: induction of histamine release and basophil accumulation by substance P.Cell Biol Toxicol. 2016 Jun;32(3):217-28. doi: 10.1007/s10565-016-9330-4. Epub 2016 May 4. Cell Biol Toxicol. 2016. PMID: 27147256
-
Substance P and the Neurokinin-1 Receptor: The New CRF.Int Rev Neurobiol. 2017;136:151-175. doi: 10.1016/bs.irn.2017.06.008. Epub 2017 Aug 18. Int Rev Neurobiol. 2017. PMID: 29056150 Review.
-
New insight into the role of substance P/neurokinin-1 receptor system in breast cancer progression and its crosstalk with microRNAs.Clin Genet. 2020 Oct;98(4):322-330. doi: 10.1111/cge.13750. Epub 2020 Apr 20. Clin Genet. 2020. PMID: 32266968 Review.
Cited by
-
Molecular Mechanisms of Neurogenic Inflammation of the Skin.Int J Mol Sci. 2023 Mar 5;24(5):5001. doi: 10.3390/ijms24055001. Int J Mol Sci. 2023. PMID: 36902434 Free PMC article. Review.
-
Altered Expression of Substance P and NK1R in CCR3+ and CD123+HLA-DR- Basophils Under Airway Allergic Conditions.Allergy Asthma Immunol Res. 2022 Nov;14(6):687-712. doi: 10.4168/aair.2022.14.6.687. Allergy Asthma Immunol Res. 2022. PMID: 36426398 Free PMC article.
-
The Itch-Scratch Cycle: A Review of the Mechanisms.Dermatol Pract Concept. 2019 Apr 30;9(2):90-97. doi: 10.5826/dpc.0902a03. eCollection 2019 Apr. Dermatol Pract Concept. 2019. PMID: 31106010 Free PMC article.
-
Involvement of Neuro-Immune Interactions in Pruritus With Special Focus on Receptor Expressions.Front Med (Lausanne). 2021 Feb 18;8:627985. doi: 10.3389/fmed.2021.627985. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33681256 Free PMC article. Review.
-
Critical Neurotransmitters in the Neuroimmune Network.Front Immunol. 2020 Aug 21;11:1869. doi: 10.3389/fimmu.2020.01869. eCollection 2020. Front Immunol. 2020. PMID: 32973771 Free PMC article. Review.
References
-
- Hijnen D, Knol EF, Gent YY, Giovannone B, Beijn SJ, Kupper TS, Bruijnzeel-Koomen CA, Clark RA. CD8+ T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-γ, IL-13, IL-17, and IL-22. J Investig Dermatol. 2013;133(4):973–979. doi: 10.1038/jid.2012.456. - DOI - PMC - PubMed
-
- Czarnowicki T, Esaki H, Gonzalez J, Malajian D, Shemer A, Noda S, Talasila S, Berry A, Gray J, Becker L, et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)(+) TH2/TH1 cell imbalance, whereas adults acquire CLA(+) TH22/TC22 cell subsets. J Allergy Clin Immunol. 2015;136(4):941–951. doi: 10.1016/j.jaci.2015.05.049. - DOI - PMC - PubMed
-
- Roesner LM, Heratizadeh A, Wieschowski S, Mittermann I, Valenta R, Eiz-Vesper B, Hennig C, Hansen G, Falk CS, Werfel T. α-NAC-specific autoreactive CD8+ T cells in atopic dermatitis are of an effector memory type and secrete IL-4 and IFN-γ. J Immunol. 2016;196(8):3245–3252. doi: 10.4049/jimmunol.1500351. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

