Hydrocephalus due to multiple ependymal malformations is caused by mutations in the MPDZ gene
- PMID: 28460636
- PMCID: PMC5412059
- DOI: 10.1186/s40478-017-0438-4
Hydrocephalus due to multiple ependymal malformations is caused by mutations in the MPDZ gene
Abstract
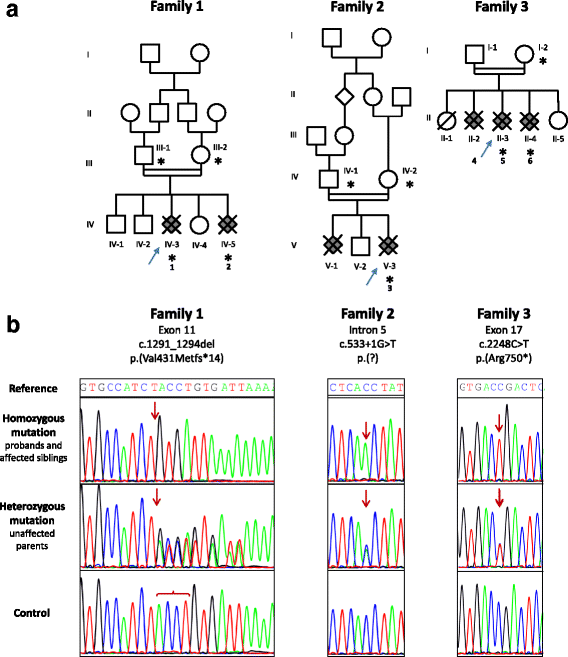
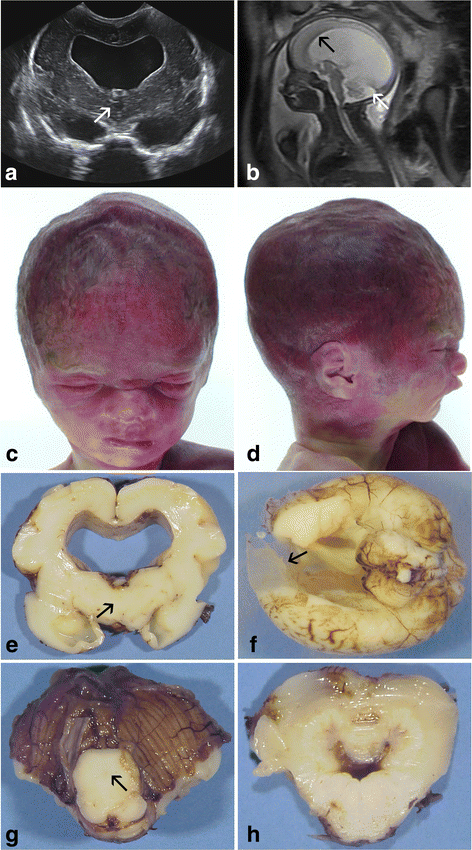
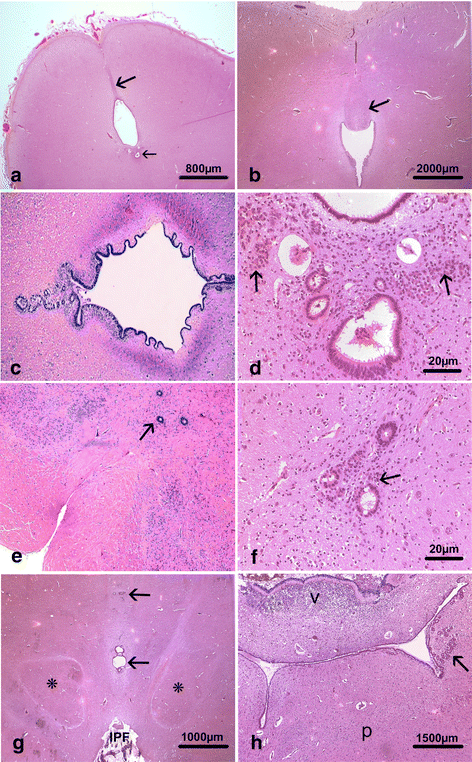
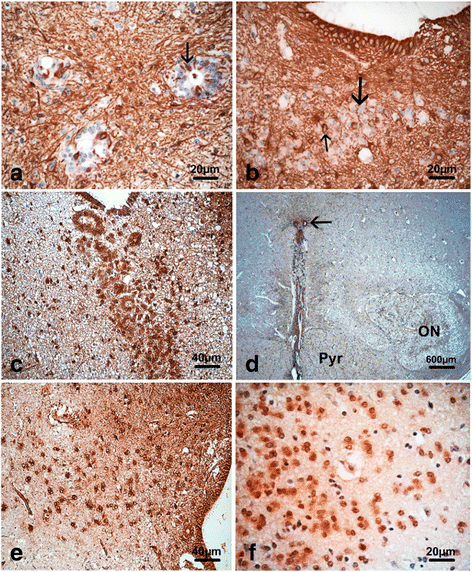
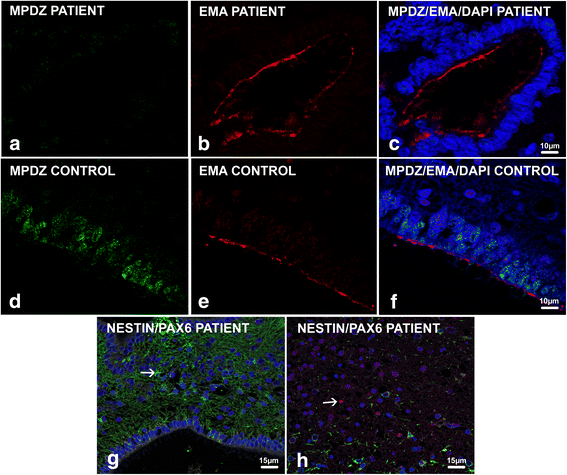
Congenital hydrocephalus is considered as either acquired due to haemorrhage, infection or neoplasia or as of developmental nature and is divided into two subgroups, communicating and obstructive. Congenital hydrocephalus is either syndromic or non-syndromic, and in the latter no cause is found in more than half of the patients. In patients with isolated hydrocephalus, L1CAM mutations represent the most common aetiology. More recently, a founder mutation has also been reported in the MPDZ gene in foetuses presenting massive hydrocephalus, but the neuropathology remains unknown. We describe here three novel homozygous null mutations in the MPDZ gene in foetuses whose post-mortem examination has revealed a homogeneous phenotype characterized by multiple ependymal malformations along the aqueduct of Sylvius, the third and fourth ventricles as well as the central canal of the medulla, consisting in multifocal rosettes with immature cell accumulation in the vicinity of ependymal lining early detached from the ventricular zone. MPDZ also named MUPP1 is an essential component of tight junctions which are expressed from early brain development in the choroid plexuses and ependyma. Alterations in the formation of tight junctions within the ependyma very likely account for the lesions observed and highlight for the first time that primary multifocal ependymal malformations of the ventricular system is genetically determined in humans. Therefore, MPDZ sequencing should be performed when neuropathological examination reveals multifocal ependymal rosette formation within the aqueduct of Sylvius, of the third and fourth ventricles and of the central canal of the medulla.
Keywords: Autosomal recessive inheritance; Foetal hydrocephalus; MPDZ pathogenic variants; Multifocal malformation of the ependyma; Neuropathology.
Figures
Similar articles
-
Neuropathological hallmarks of fetal hydrocephalus linked to CCDC88C pathogenic variants.Acta Neuropathol Commun. 2021 Jun 6;9(1):104. doi: 10.1186/s40478-021-01207-5. Acta Neuropathol Commun. 2021. PMID: 34092257 Free PMC article.
-
Bi-allelic variations in CRB2, encoding the crumbs cell polarity complex component 2, lead to non-communicating hydrocephalus due to atresia of the aqueduct of sylvius and central canal of the medulla.Acta Neuropathol Commun. 2023 Feb 20;11(1):29. doi: 10.1186/s40478-023-01519-8. Acta Neuropathol Commun. 2023. PMID: 36803301 Free PMC article.
-
Loss of Mpdz impairs ependymal cell integrity leading to perinatal-onset hydrocephalus in mice.EMBO Mol Med. 2017 Jul;9(7):890-905. doi: 10.15252/emmm.201606430. EMBO Mol Med. 2017. PMID: 28500065 Free PMC article.
-
The secretory ependymal cells of the subcommissural organ: which role in hydrocephalus?Int J Biochem Cell Biol. 2007;39(3):463-8. doi: 10.1016/j.biocel.2006.10.021. Epub 2006 Nov 2. Int J Biochem Cell Biol. 2007. PMID: 17150405 Review.
-
Riding the wave of ependymal cilia: genetic susceptibility to hydrocephalus in primary ciliary dyskinesia.J Neurosci Res. 2013 Sep;91(9):1117-32. doi: 10.1002/jnr.23238. Epub 2013 May 17. J Neurosci Res. 2013. PMID: 23686703 Review.
Cited by
-
Progress in brain barriers and brain fluid research in 2017.Fluids Barriers CNS. 2018 Feb 2;15(1):6. doi: 10.1186/s12987-018-0091-8. Fluids Barriers CNS. 2018. PMID: 29391031 Free PMC article.
-
Inference of Diagnostic Markers and Therapeutic Targets From CSF Proteomics for the Treatment of Hydrocephalus.Front Cell Neurosci. 2020 Oct 21;14:576028. doi: 10.3389/fncel.2020.576028. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33192320 Free PMC article. No abstract available.
-
Microglia activated by microbial neuraminidase contributes to ependymal cell death.Fluids Barriers CNS. 2021 Mar 23;18(1):15. doi: 10.1186/s12987-021-00249-0. Fluids Barriers CNS. 2021. PMID: 33757539 Free PMC article.
-
Ependymal cells: roles in central nervous system infections and therapeutic application.J Neuroinflammation. 2024 Oct 9;21(1):255. doi: 10.1186/s12974-024-03240-2. J Neuroinflammation. 2024. PMID: 39385253 Free PMC article. Review.
-
Cerebral furin deficiency causes hydrocephalus in mice.Genes Dis. 2023 Jul 8;11(3):101009. doi: 10.1016/j.gendis.2023.04.037. eCollection 2024 May. Genes Dis. 2023. PMID: 38292192 Free PMC article.
References
-
- Adle-Biassette H, Saugier-Veber P, Fallet-Bianco C, Delezoide AL, Razavi F, Drouot N, Bazin A, Beaufrère AM, Bessières B, Blesson S, Bucourt M, Carles D, Devisme L, Dijoud F, Fabre B, Fernandez C, Gaillard D, Gonzales M, Jossic F, Joubert M, Laurent N, Leroy B, Loeuillet L, Loget P, Marcorelles P, Martinovic J, Perez MJ, Satge D, Sinico M, Tosi M, Benichou J, Gressens P, Frebourg T, Laquerrière A. Neuropathological review of 138 cases genetically tested for X-linked hydrocephalus: evidence for closely related clinical entities of unknown molecular bases. Acta Neuropathol. 2013;126(3):427–42. doi: 10.1007/s00401-013-1146-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

