Core outcome sets for research and clinical practice
- PMID: 28460714
- PMCID: PMC5537457
- DOI: 10.1016/j.bjpt.2017.03.001
Core outcome sets for research and clinical practice
Abstract
Background: This masterclass introduces the topic of core outcome sets, describing rationale and methods for developing them, and providing some examples that are relevant for clinical research and practice.
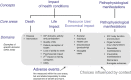
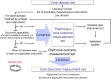
Method: A core outcome set is a minimum consensus-based set of outcomes that should be measured and reported in all clinical trials for a specific health condition and/or intervention. Issues surrounding outcome assessment, such as selective reporting and inconsistency across studies, can be addressed by the development of a core set. As suggested by key initiatives in this field (i.e. OMERACT and COMET), the development requires achieving consensus on: (1) core outcome domains and (2) core outcome measurement instruments. Different methods can be used to reach consensus, including: literature systematic reviews to inform the process, qualitative research with clinicians and patients, group discussions (e.g. nominal group technique), and structured surveys (e.g. Delphi technique). Various stakeholders should be involved in the process, with particular attention to patients.
Results and conclusions: Several COSs have been developed for musculoskeletal conditions including a longstanding one for low back pain, IMMPACT recommendations on outcomes for chronic pain, and OMERACT COSs for hip, knee and hand osteoarthritis. There is a lack of COSs for neurological, geriatric, cardio-respiratory and pediatric conditions, therefore, future research could determine the value of developing COSs for these conditions.
Keywords: Core outcome set; Effectiveness; Interventions; Musculoskeletal pain.
Copyright © 2017 Associação Brasileira de Pesquisa e Pós-Graduação em Fisioterapia. Publicado por Elsevier Editora Ltda. All rights reserved.
Figures
Similar articles
-
A core outcome set for clinical trials on non-specific low back pain: study protocol for the development of a core domain set.Trials. 2014 Dec 26;15:511. doi: 10.1186/1745-6215-15-511. Trials. 2014. PMID: 25540987 Free PMC article.
-
Relevant domains, core outcome sets and measurements for implant dentistry clinical trials: The Implant Dentistry Core Outcome Set and Measurement (ID-COSM) international consensus report.Clin Oral Implants Res. 2023 May;34 Suppl 25:4-21. doi: 10.1111/clr.14074. Clin Oral Implants Res. 2023. PMID: 37232121
-
IMMPACT-recommended outcome measures and tools of assessment in burning mouth syndrome RCTs: an international Delphi survey protocol.Trials. 2020 Aug 12;21(1):711. doi: 10.1186/s13063-020-04640-4. Trials. 2020. PMID: 32787910 Free PMC article.
-
Outcome Measures in Rheumatology - Interventions for medication Adherence (OMERACT-Adherence) Core Domain Set for Trials of Interventions for Medication Adherence in Rheumatology: 5 Phase Study Protocol.Trials. 2018 Mar 27;19(1):204. doi: 10.1186/s13063-018-2565-z. Trials. 2018. PMID: 29587864 Free PMC article.
-
Core Outcome Measures in Effectiveness Trials (COMET) initiative: protocol for an international Delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a 'core outcome set'.Trials. 2014 Jun 25;15:247. doi: 10.1186/1745-6215-15-247. Trials. 2014. PMID: 24962012 Free PMC article. Review.
Cited by
-
Women's experiences of maternity care in England: preliminary development of a standard measure.BMC Pregnancy Childbirth. 2019 May 14;19(1):167. doi: 10.1186/s12884-019-2284-9. BMC Pregnancy Childbirth. 2019. PMID: 31088487 Free PMC article.
-
A low-cost easily implementable physiotherapy intervention clinically improves gait implying better adaptation to lower limb prosthesis: a randomized clinical trial.Sci Rep. 2021 Oct 27;11(1):21228. doi: 10.1038/s41598-021-00686-9. Sci Rep. 2021. PMID: 34707169 Free PMC article. Clinical Trial.
-
Selecting Review Outcomes for Systematic Reviews of Public Health Interventions.Am J Public Health. 2021 Mar;111(3):465-470. doi: 10.2105/AJPH.2020.306061. Epub 2021 Jan 21. Am J Public Health. 2021. PMID: 33476230 Free PMC article.
-
Laparoscopic cholecystectomy versus conservative management for adults with uncomplicated symptomatic gallstones: the C-GALL RCT.Health Technol Assess. 2024 Jun;28(26):1-151. doi: 10.3310/MNBY3104. Health Technol Assess. 2024. PMID: 38943314 Free PMC article. Clinical Trial.
-
Investigating the Secondary Use of Clinical Research Data: Protocol for a Mixed Methods Study.JMIR Res Protoc. 2023 Mar 6;12:e44875. doi: 10.2196/44875. JMIR Res Protoc. 2023. PMID: 36877564 Free PMC article.
References
-
- Grobbee D.E., Hoes A.W. Jones & Bartlett Learning; 2009. Clinical Epidemiology: Principles, Methods, and Applications for Clinical Research.
-
- Tugwell P., Boers M. OMERACT conference on outcome measures in rheumatoid arthritis clinical trials: introduction. J Rheumatol. 1993;20(3):528. - PubMed
-
- Kalyoncu U., Dougados M., Daures J., Gossec L. Reporting of patient-reported outcomes in recent trials in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2009;68(2):183–190. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

