Efficacy and safety of ridinilazole compared with vancomycin for the treatment of Clostridium difficile infection: a phase 2, randomised, double-blind, active-controlled, non-inferiority study
- PMID: 28461207
- PMCID: PMC5483507
- DOI: 10.1016/S1473-3099(17)30235-9
Efficacy and safety of ridinilazole compared with vancomycin for the treatment of Clostridium difficile infection: a phase 2, randomised, double-blind, active-controlled, non-inferiority study
Abstract
Background: Clostridium difficile infection is the most common health-care-associated infection in the USA. We assessed the safety and efficacy of ridinilazole versus vancomycin for treatment of C difficile infection.
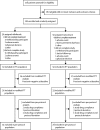
Methods: We did a phase 2, randomised, double-blind, active-controlled, non-inferiority study. Participants with signs and symptoms of C difficile infection and a positive diagnostic test result were recruited from 33 centres in the USA and Canada and randomly assigned (1:1) to receive oral ridinilazole (200 mg every 12 h) or oral vancomycin (125 mg every 6 h) for 10 days. The primary endpoint was achievement of a sustained clinical response, defined as clinical cure at the end of treatment and no recurrence within 30 days, which was used to establish non-inferiority (15% margin) of ridinilazole versus vancomycin. The primary efficacy analysis was done on a modified intention-to-treat population comprising all individuals with C difficile infection confirmed by the presence of free toxin in stool who were randomly assigned to receive one or more doses of the study drug. The study is registered with ClinicalTrials.gov, number NCT02092935.
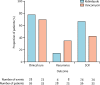
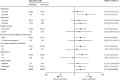
Findings: Between June 26, 2014, and August 31, 2015, 100 patients were recruited; 50 were randomly assigned to receive ridinilazole and 50 to vancomycin. 16 patients did not complete the study, and 11 discontinued treatment early. The primary efficacy analysis included 69 patients (n=36 in the ridinilazole group; n=33 in the vancomycin group). 24 of 36 (66·7%) patients in the ridinilazole group versus 14 of 33 (42·4%) of those in the vancomycin group had a sustained clinical response (treatment difference 21·1%, 90% CI 3·1-39·1, p=0·0004), establishing the non-inferiority of ridinilazole and also showing statistical superiority at the 10% level. Ridinilazole was well tolerated, with an adverse event profile similar to that of vancomycin: 82% (41 of 50) of participants reported adverse events in the ridinilazole group and 80% (40 of 50) in the vancomycin group. There were no adverse events related to ridinilazole that led to discontinuation.
Interpretation: Ridinilazole is a targeted-spectrum antimicrobial that shows potential in treatment of initial C difficile infection and in providing sustained benefit through reduction in disease recurrence. Further clinical development is warranted.
Funding: Wellcome Trust and Summit Therapeutics.
Copyright © 2017 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Expanding the armamentarium for the treatment of Clostridium difficile infection.Lancet Infect Dis. 2017 Jul;17(7):678-680. doi: 10.1016/S1473-3099(17)30237-2. Epub 2017 Apr 28. Lancet Infect Dis. 2017. PMID: 28461208 No abstract available.
-
Ridinilazole-a novel antibiotic for treatment of Clostridium difficile infection.J Thorac Dis. 2018 Jan;10(1):118-120. doi: 10.21037/jtd.2017.12.117. J Thorac Dis. 2018. PMID: 29600036 Free PMC article. No abstract available.
References
-
- Davies KA, Longshaw CM, Davis GL. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID) Lancet Infect Dis. 2014;14:1208–1219. - PubMed
-
- Bauer MP, Notermans DW, van Benthem BH, for the ECDIS Study Group Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377:63–73. - PubMed
-
- Magee G, Strauss ME, Thomas SM, Brown H, Baumer D, Broderick KC. Impact of Clostridium difficile-associated diarrhea on acute care length of stay, hospital costs, and readmission: a multicenter retrospective study of inpatients, 2009–2011. Am J Infect Control. 2015;43:1148–1153. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical