Insights from clinical research completed during the west Africa Ebola virus disease epidemic
- PMID: 28461209
- PMCID: PMC5856335
- DOI: 10.1016/S1473-3099(17)30234-7
Insights from clinical research completed during the west Africa Ebola virus disease epidemic
Abstract
The west Africa Ebola virus disease (EVD) epidemic was extraordinary in scale. Now that the epidemic has ended, it is a relevant time to examine published studies with direct relevance to clinical care and, more broadly, to examine the implications of the clinical research response mounted. Clinically relevant research includes literature detailing risk factors for and clinical manifestations of EVD, laboratory and other investigation findings in patients, experimental vaccine and therapeutic clinical trials, and analyses of survivor syndrome. In this Review, we discuss new insights from patient-oriented research completed during the west Africa epidemic, identify ongoing knowledge gaps, and suggest priorities for future research.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
We declare no competing interests. The authors were investigators for two clinical trials described in this Review (TKM-130803 and brincidofovir trials).
Figures
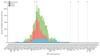
Comment in
-
Initiation and publication time-lags of treatment trials for Ebola virus disease.Lancet Infect Dis. 2018 Jan;18(1):28-29. doi: 10.1016/S1473-3099(17)30698-9. Epub 2017 Dec 20. Lancet Infect Dis. 2018. PMID: 29303737 No abstract available.
References
-
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371:1418–25. - PubMed
-
- WHO. Statement on the 1st meeting of the IHR Emergency Committee on the 2014 Ebola outbreak in west Africa. [accessed March 1, 2016];2014 http://www.who.int/mediacentre/news/statements/2014/ebola-20140808/en/
-
- WHO. Ebola situation report—30 March 2016. [accessed March 30, 2016]; http://apps.who.int/ebola/current-situation/ebola-situation-report-30-ma....
-
- UN Economic Commission for Africa. Socio-economic impacts of Ebola on Africa (Revised edition) [accessed Oct 11, 2016];2015 http://www.uneca.org/sites/default/files/PublicationFiles/eca_ebola_repo....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

