CREBH Maintains Circadian Glucose Homeostasis by Regulating Hepatic Glycogenolysis and Gluconeogenesis
- PMID: 28461393
- PMCID: PMC5492176
- DOI: 10.1128/MCB.00048-17
CREBH Maintains Circadian Glucose Homeostasis by Regulating Hepatic Glycogenolysis and Gluconeogenesis
Abstract
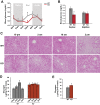
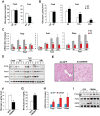
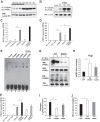
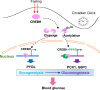
Cyclic AMP-responsive element binding protein, hepatocyte specific (CREBH), is a liver-enriched, endoplasmic reticulum-tethered transcription factor known to regulate the hepatic acute-phase response and lipid homeostasis. In this study, we demonstrate that CREBH functions as a circadian transcriptional regulator that plays major roles in maintaining glucose homeostasis. The proteolytic cleavage and posttranslational acetylation modification of CREBH are regulated by the circadian clock. Functionally, CREBH is required in order to maintain circadian homeostasis of hepatic glycogen storage and blood glucose levels. CREBH regulates the rhythmic expression of the genes encoding the rate-limiting enzymes for glycogenolysis and gluconeogenesis, including liver glycogen phosphorylase (PYGL), phosphoenolpyruvate carboxykinase 1 (PCK1), and the glucose-6-phosphatase catalytic subunit (G6PC). CREBH interacts with peroxisome proliferator-activated receptor α (PPARα) to synergize its transcriptional activities in hepatic gluconeogenesis. The acetylation of CREBH at lysine residue 294 controls CREBH-PPARα interaction and synergy in regulating hepatic glucose metabolism in mice. CREBH deficiency leads to reduced blood glucose levels but increases hepatic glycogen levels during the daytime or upon fasting. In summary, our studies revealed that CREBH functions as a key metabolic regulator that controls glucose homeostasis across the circadian cycle or under metabolic stress.
Keywords: CREBH; circadian metabolism; gluconeogenesis; glucose metabolism; glycogenolysis; liver metabolism; nuclear receptor; transcriptional regulation.
Copyright © 2017 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
