Microtubule-Based Transport and the Distribution, Tethering, and Organization of Organelles
- PMID: 28461574
- PMCID: PMC5411697
- DOI: 10.1101/cshperspect.a025817
Microtubule-Based Transport and the Distribution, Tethering, and Organization of Organelles
Abstract
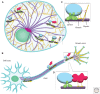
SUMMARYMicrotubules provide long tracks along which a broad range of organelles and vesicles are transported by kinesin and dynein motors. Motor protein complexes also tether cargoes to cytoskeletal filaments, helping facilitate their interaction and communication. The generation of biochemically distinct microtubule subpopulations allows subsets of motors to recognize a given microtubule identity, allowing further organization within the cytoplasm. Both transport and tethering are spatiotemporally regulated through multiple modes, including acute modification of both motor-cargo and motor-track associations by various physiological signals. Strict regulation of intracellular transport is particularly important in specialized cell types such as neurons. Here, we review general mechanisms by which cargo transport is controlled and also highlight examples of transport regulated by multiple mechanisms.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
