Genetic risk for schizophrenia and psychosis in Alzheimer disease
- PMID: 28461698
- PMCID: PMC5668212
- DOI: 10.1038/mp.2017.81
Genetic risk for schizophrenia and psychosis in Alzheimer disease
Erratum in
-
Correction: Genetic risk for schizophrenia and psychosis in Alzheimer disease.Mol Psychiatry. 2020 Nov;25(11):3109-3111. doi: 10.1038/s41380-019-0373-9. Mol Psychiatry. 2020. PMID: 30862939
Abstract
Psychotic symptoms, defined as the occurrence of delusions or hallucinations, are frequent in Alzheimer disease (AD), affecting ~40 to 60% of individuals with AD (AD with psychosis (AD+P)). In comparison with AD subjects without psychosis, AD+P subjects have more rapid cognitive decline and poor outcomes. Prior studies have estimated the heritability of psychosis in AD at 61%, but the underlying genetic sources of this risk are not known. We evaluated a Discovery Cohort of 2876 AD subjects with (N=1761) or without psychosis (N=1115). All subjects were genotyped using a custom genotyping array designed to evaluate single-nucleotide polymorphisms (SNPs) with evidence of genetic association with AD+P and include SNPs affecting or putatively affecting risk for schizophrenia and AD. Results were replicated in an independent cohort of 2194 AD subjects with (N=734) or without psychosis (N=1460). We found that AD+P is associated with polygenic risk for a set of novel loci and inversely associated with polygenic risk for schizophrenia. Among the biologic pathways identified by the associations of schizophrenia SNPs with AD+P are endosomal trafficking, autophagy and calcium channel signaling. To the best of our knowledge, these findings provide the first clear demonstration that AD+P is associated with common genetic variation. In addition, they provide an unbiased link between polygenic risk for schizophrenia and a lower risk of psychosis in AD. This provides an opportunity to leverage progress made in identifying the biologic effects of schizophrenia alleles to identify novel mechanisms protecting against more rapid cognitive decline and psychosis risk in AD.
Conflict of interest statement
Figures
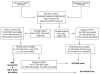
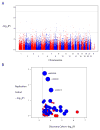

References
-
- Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005;162:2022–30. - PubMed
Publication types
MeSH terms
Grants and funding
- R37 MH057881/MH/NIMH NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- R01 MH093723/MH/NIMH NIH HHS/United States
- U24 AG056270/AG/NIA NIH HHS/United States
- R01 MH057881/MH/NIMH NIH HHS/United States
- MR/L023784/1/MRC_/Medical Research Council/United Kingdom
- P50 AG005133/AG/NIA NIH HHS/United States
- R01 AG041797/AG/NIA NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- MR/L023784/2/MRC_/Medical Research Council/United Kingdom
- G0300429/MRC_/Medical Research Council/United Kingdom
- U01 AG016976/AG/NIA NIH HHS/United States
- R01 AG030653/AG/NIA NIH HHS/United States
- R01 AG027224/AG/NIA NIH HHS/United States
- I01 BX000452/BX/BLRD VA/United States
- R01 AG041718/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

