Combining nano-physical and computational investigations to understand the nature of "aging" in dermal collagen
- PMID: 28461747
- PMCID: PMC5407446
- DOI: 10.2147/IJN.S121400
Combining nano-physical and computational investigations to understand the nature of "aging" in dermal collagen
Abstract
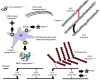
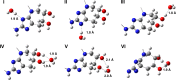
The extracellular matrix of the dermis is a complex, dynamic system with the various dermal components undergoing individual physiologic changes as we age. Age-related changes in the physical properties of collagen were investigated in particular by measuring the effect of aging, most likely due to the accumulation of advanced glycation end product (AGE) cross-links, on the nanomechanical properties of the collagen fibril using atomic force microscope nano-indentation. An age-related decrease in the Young's modulus of the transverse fibril was observed (from 8.11 to 4.19 GPa in young to old volunteers, respectively, P<0.001). It is proposed that this is due to a change in the fibril density caused by age-related differences in water retention within the fibrils. The new collagen-water interaction mechanism was verified by electronic structure calculations, showing it to be energetically feasible.
Keywords: advanced glycation end products; aging; atomic force microscopy; collagen; nanomechanics; nanotechnology.
Conflict of interest statement
Disclosure Helen L Birch received BBSRC grant BB/K007785/1 which supported this project. Anne Potter, Marion Ghibaudo, and Ramona Enea Casse are employees of L’Oréal. The authors report no other conflicts of interest in this work.
Figures




References
-
- Select Committee on Public Service and Demographic Change Report of Session 2012–13 M . Ready for Ageing? London, UK: The Stationery Office Limited; Authority of the House of Lords; 2013. Mar 5, 2013. HL Paper 140.
-
- ONS . 2010-based national population projections lifetable template. England and Wales: Office of National Statistics; 2011.
-
- Lovell CR, Smolenski KA, Duance VC, Light ND, Young S, Dyson M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br J Dermatol. 1987;117(4):419–428. - PubMed
-
- Jeanmaire C, Danoux L, Pauly G. Glycation during human dermal intrinsic and actinic ageing: an in vivo and in vitro model study. Br J Dermatol. 2001;145(1):10–18. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

