Comparative toxicity study on classical and modified version of Jawarish Jalinoos (a traditional Unani formulation) in rats
- PMID: 28462146
- PMCID: PMC5395683
- DOI: 10.1016/j.imr.2017.01.001
Comparative toxicity study on classical and modified version of Jawarish Jalinoos (a traditional Unani formulation) in rats
Abstract
Background: Jawarish Jalinoos (JJ) is a classical semisolid traditional Unani formulation clinically used for the treatment of weakness of vital organs, liver, and stomach. Although JJ has been widely used clinically for several decades, no scientific report is available for its safety.
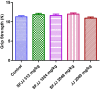
Methods: JJ and its sugar-free tablet version (SFJJ; formulated to target diabetic population) were assessed for safety in rats. Ninety-day repeated dose oral toxicity study was performed as per the Organisation for Economic Co-operation and Development Guideline 408. JJ was orally administered at the dose of 2000 mg/kg bw/d, whereas SFJJ was orally administered at the doses of 506 mg/kg body weight (bw)/d, 1012 mg/kg bw/d, and 2024 mg/kg bw/d for 90 days. The animals were periodically observed for clinical signs of toxicity, mortality, morbidity, body weight changes, and feed consumption. At the end of the study, hematology, clinical biochemistry, electrolytes, gross pathology, relative organ weight, and histological examination were performed.
Results: Treatment with SFJJ and JJ showed no significant differences in body weight gain, feed consumption, hematology, clinical biochemistry, and serum electrolytes. No gross pathological findings and differences in relative organ weights were observed between control and drug treated rats. Histological examination revealed no toxicologically significant abnormalities related with SFJJ or JJ treatment.
Conclusion: The 90-day repeated dose oral toxicity study demonstrates that the no observed adverse effect level of SFJJ and JJ is greater than 2024 mg/kg bw/d and 2000 mg/kg bw/d (p.o.) in rats, respectively. Both formulations were found to be safe up to the tested dose levels and experimental conditions, and therefore safe for clinical use as specified in the literature.
Keywords: Jawarish Jalinoos; Unani; polyherbal formulation; rat; safety evaluation.
Figures






Similar articles
-
NTP technical report on the toxicity studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice.Toxic Rep Ser. 1995 Apr;30:1-G5. Toxic Rep Ser. 1995. PMID: 12209194
-
NTP Toxicology and Carcinogenesis Studies of 4,4'-Thiobis(6- t -butyl- m -cresol) (CAS No. 96-69-5) in F344/N Rats and B6C3F1 Mice (Feed Studies).Natl Toxicol Program Tech Rep Ser. 1994 Dec;435:1-288. Natl Toxicol Program Tech Rep Ser. 1994. PMID: 12595928
-
Acute and subacute toxicity assessment of Madhulai Manappagu (Siddha herbal syrup formulation) in animal model.J Complement Integr Med. 2021 Apr 5;19(1):9-18. doi: 10.1515/jcim-2020-0327. J Complement Integr Med. 2021. PMID: 33818028
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
-
Characterisation and toxicological assessment of Neutral Methacrylate Copolymer for GRAS evaluation.Regul Toxicol Pharmacol. 2013 Dec;67(3):392-408. doi: 10.1016/j.yrtph.2013.08.019. Epub 2013 Sep 5. Regul Toxicol Pharmacol. 2013. PMID: 24012708 Review.
References
-
- AYUSH . Department of AYUSH, Ministry of Health and Family Welfare, Government of India; 2013. Unani system of medicine—the science of health and healing New Delh. p. 11.
-
- Rais-ur-Rahman . Yojana. 2015 June. Unani medicine: the art of health and healing; pp. 43–47. Available from: http://iasscore.in/pdf/yojna/UNANI%20Medicine.pdf. Accessed December 9, 2016.
-
- CCRUM (Central Council for Research in Unani Medicine) In: National Formulary of Unani Medicine (NFUM) 1 st reprint ed. Department of AYUSH MoHFW, Government of India, editor. CCRUM; New Delhi: 2006. p. 100.
-
- Khan S. Daftar-ul-Masih; New Delhi: 1937. Ilaj-ul-Amraz (Urdu Translation by Hakeem Kabiruddin) p. 356.
-
- Hasan M. Siddiqui Press Delhi; Delhi: 1904. Qarabadeen-E-Azam (Urdu) p. 24.
LinkOut - more resources
Full Text Sources
Other Literature Sources

