Variations in brain defects result from cellular mosaicism in the activation of heat shock signalling
- PMID: 28462912
- PMCID: PMC5418582
- DOI: 10.1038/ncomms15157
Variations in brain defects result from cellular mosaicism in the activation of heat shock signalling
Abstract
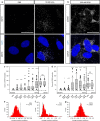
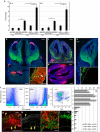
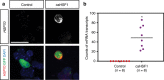
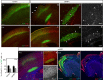
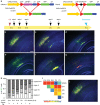
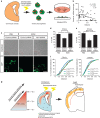
Repetitive prenatal exposure to identical or similar doses of harmful agents results in highly variable and unpredictable negative effects on fetal brain development ranging in severity from high to little or none. However, the molecular and cellular basis of this variability is not well understood. This study reports that exposure of mouse and human embryonic brain tissues to equal doses of harmful chemicals, such as ethanol, activates the primary stress response transcription factor heat shock factor 1 (Hsf1) in a highly variable and stochastic manner. While Hsf1 is essential for protecting the embryonic brain from environmental stress, excessive activation impairs critical developmental events such as neuronal migration. Our results suggest that mosaic activation of Hsf1 within the embryonic brain in response to prenatal environmental stress exposure may contribute to the resulting generation of phenotypic variations observed in complex congenital brain disorders.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Detection of vulnerable neurons damaged by environmental insults in utero.Proc Natl Acad Sci U S A. 2017 Feb 28;114(9):2367-2372. doi: 10.1073/pnas.1620641114. Epub 2017 Jan 25. Proc Natl Acad Sci U S A. 2017. PMID: 28123061 Free PMC article.
-
Receptor-interacting protein 140 as a co-repressor of Heat Shock Factor 1 regulates neuronal stress response.Cell Death Dis. 2017 Dec 12;8(12):3203. doi: 10.1038/s41419-017-0008-5. Cell Death Dis. 2017. PMID: 29233969 Free PMC article.
-
Comparative approaches to understanding thyroid hormone regulation of neurogenesis.Mol Cell Endocrinol. 2017 Dec 25;459:104-115. doi: 10.1016/j.mce.2017.05.020. Epub 2017 May 22. Mol Cell Endocrinol. 2017. PMID: 28545819 Review.
-
Modulation of Heat Shock Factor 1 Activity through Silencing of Ser303/Ser307 Phosphorylation Supports a Metabolic Program Leading to Age-Related Obesity and Insulin Resistance.Mol Cell Biol. 2018 Aug 28;38(18):e00095-18. doi: 10.1128/MCB.00095-18. Print 2018 Sep 15. Mol Cell Biol. 2018. PMID: 29941492 Free PMC article.
-
Cellular stress mechanisms of prenatal maternal stress: Heat shock factors and oxidative stress.Neurosci Lett. 2019 Sep 14;709:134368. doi: 10.1016/j.neulet.2019.134368. Epub 2019 Jul 9. Neurosci Lett. 2019. PMID: 31299286 Free PMC article. Review.
Cited by
-
Trans Species RNA Activity: Sperm RNA of the Father of an Autistic Child Programs Glial Cells and Behavioral Disorders in Mice.Biomolecules. 2024 Feb 7;14(2):201. doi: 10.3390/biom14020201. Biomolecules. 2024. PMID: 38397438 Free PMC article.
-
Detection of local and remote cellular damage caused by spinal cord and peripheral nerve injury using a heat shock signaling reporter system.IBRO Rep. 2018 Nov 6;5:91-98. doi: 10.1016/j.ibror.2018.11.003. eCollection 2018 Dec. IBRO Rep. 2018. PMID: 30480161 Free PMC article.
-
Caspase-3 Inhibition toward Perinatal Protection of the Developing Brain from Environmental Stress.Dev Neurosci. 2023;45(2):66-75. doi: 10.1159/000529125. Epub 2023 Jan 13. Dev Neurosci. 2023. PMID: 36642064 Free PMC article. Review.
-
Prenatal Environment That Affects Neuronal Migration.Front Cell Dev Biol. 2019 Jul 17;7:138. doi: 10.3389/fcell.2019.00138. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31380373 Free PMC article. Review.
-
Tracking the Activation of Heat Shock Signaling in Cellular Protection and Damage.Cells. 2022 May 5;11(9):1561. doi: 10.3390/cells11091561. Cells. 2022. PMID: 35563865 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

