A conserved hexanucleotide motif is important in UV-inducible promoters in Sulfolobus acidocaldarius
- PMID: 28463103
- PMCID: PMC5817253
- DOI: 10.1099/mic.0.000455
A conserved hexanucleotide motif is important in UV-inducible promoters in Sulfolobus acidocaldarius
Abstract
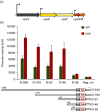
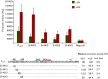
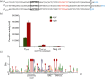
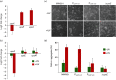
Upon DNA damage, Sulfolobales exhibit a global gene regulatory response resulting in the expression of DNA transfer and repair proteins and the repression of the cell division machinery. Because the archaeal DNA damage response is still poorly understood, we investigated the promoters of the highly induced ups operon. Ups pili are involved in cellular aggregation and DNA exchange between cells. With LacS reporter gene assays we identified a conserved, non-palindromic hexanucleotide motif upstream of the ups core promoter elements to be essential for promoter activity. Substitution of this cis regulatory motif in the ups promoters resulted in abolishment of cellular aggregation and reduced DNA transfer. By screening the Sulfolobus acidocaldarius genome we identified a total of 214 genes harbouring the hexanucleotide motif in their respective promoter regions. Many of these genes were previously found to be regulated upon UV light treatment. Given the fact that the identified motif is conserved among S. acidocaldarius and Sulfolobus tokodaii promoters, we speculate that a common regulatory mechanism is present in these two species in response to DNA-damaging conditions.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
-
- Karr EA. Advances in Applied Microbiology. 1st ed. vol. 89. Elsevier Inc; 2014. Transcription regulation in the third domain; pp. 101–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

