T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas
- PMID: 28463396
- PMCID: PMC5568958
- DOI: 10.1002/cncr.30726
T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas
Abstract
Background: Patients with metastatic sarcomas have poor outcomes and although the disease may be amenable to immunotherapies, information regarding the immunologic profiles of soft tissue sarcoma (STS) subtypes is limited.
Methods: The authors identified patients with the common STS subtypes: leiomyosarcoma, undifferentiated pleomorphic sarcoma (UPS), synovial sarcoma (SS), well-differentiated/dedifferentiated liposarcoma, and myxoid/round cell liposarcoma. Gene expression, immunohistochemistry for programmed cell death protein (PD-1) and programmed death-ligand 1 (PD-L1), and T-cell receptor Vβ gene sequencing were performed on formalin-fixed, paraffin-embedded tumors from 81 patients. Differences in liposarcoma subsets also were evaluated.
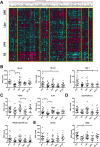
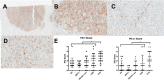
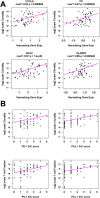
Results: UPS and leiomyosarcoma had high expression levels of genes related to antigen presentation and T-cell infiltration. UPS were found to have higher levels of PD-L1 (P≤.001) and PD-1 (P≤.05) on immunohistochemistry and had the highest T-cell infiltration based on T-cell receptor sequencing, significantly more than SS, which had the lowest (P≤.05). T-cell infiltrates in UPS also were more oligoclonal compared with SS and liposarcoma (P≤.05). A model adjusted for STS histologic subtype found that for all sarcomas, T-cell infiltration and clonality were highly correlated with PD-1 and PD-L1 expression levels (P≤.01).
Conclusions: In the current study, the authors provide the most detailed overview of the immune microenvironment in sarcoma subtypes to date. UPS, which is a more highly mutated STS subtype, provokes a substantial immune response, suggesting that it may be well suited to treatment with immune checkpoint inhibitors. The SS and liposarcoma subsets are less mutated but do express immunogenic self-antigens, and therefore strategies to improve antigen presentation and T-cell infiltration may allow for successful immunotherapy in patients with these diagnoses. Cancer 2017;123:3291-304. © 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the Creative Commons Attribution NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Keywords: T-cell receptors; gene expression; immunotherapy; leiomyosarcoma; liposarcoma; pleomorphic; programmed cell death protein (PD-1); programmed death-ligand 1 (PD-L1); sarcoma.
© 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Figures

References
-
- June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med. 2015;7:280ps7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

