A simple answer to complex questions: Caenorhabditis elegans as an experimental model for examining the DNA damage response and disease genes
- PMID: 28463453
- PMCID: PMC5562273
- DOI: 10.1002/jcp.25979
A simple answer to complex questions: Caenorhabditis elegans as an experimental model for examining the DNA damage response and disease genes
Abstract
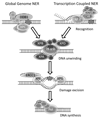
The genetic information is constantly challenged by genotoxic attacks. DNA repair mechanisms evolved early in evolution and recognize and remove the various lesions. A complex network of DNA damage responses (DDR) orchestrates a variety of physiological adaptations to the presence of genome instability. Erroneous repair or malfunctioning of the DDR causes cancer development and the accumulation of DNA lesions drives the aging process. For understanding the complex DNA repair and DDR mechanisms it is pivotal to employ simple metazoan as model systems. The nematode Caenorhabditis elegans has become a well-established and popular experimental organism that allows dissecting genome stability mechanisms in dynamic and differentiated tissues and under physiological conditions. We provide an overview of the distinct advantages of the nematode system for studying DDR and provide a range of currently applied methodologies.
Keywords: C. elegans; DNA damage response; DNA repair; aging; genome stability.
© 2017 Wiley Periodicals, Inc.
Figures




References
-
- Alpi A, Pasierbek P, Gartner A, Loidl J. Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma. 2003;112:6–16. - PubMed
-
- Antebi A. Ageing: when less is more. Nature. 2007;447:536–537. - PubMed
-
- Blum ES, Driscoll M, Shaham S. Noncanonical cell death programs in the nematode Caenorhabditis elegans. Cell Death Differ. 2008;15:1124–1131. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

