A Functional Toll-Interacting Protein Variant Is Associated with Bacillus Calmette-Guérin-Specific Immune Responses and Tuberculosis
- PMID: 28463648
- PMCID: PMC5564674
- DOI: 10.1164/rccm.201611-2346OC
A Functional Toll-Interacting Protein Variant Is Associated with Bacillus Calmette-Guérin-Specific Immune Responses and Tuberculosis
Abstract
Rationale: The molecular mechanisms that regulate tuberculosis susceptibility and bacillus Calmette-Guérin (BCG)-induced immunity are mostly unknown. However, induction of the adaptive immune response is a critical step in host control of Mycobacterium tuberculosis. Toll-interacting protein (TOLLIP) is a ubiquitin-binding protein that regulates innate immune responses, including Toll-like receptor signaling, which initiate adaptive immunity. TOLLIP variation is associated with susceptibility to tuberculosis, but the mechanism by which it regulates tuberculosis immunity is poorly understood.
Objectives: To identify functional TOLLIP variants and evaluate the role of TOLLIP variation on innate and adaptive immune responses to mycobacteria and susceptibility to tuberculosis.
Methods: We used human cellular immunology approaches to characterize the role of a functional TOLLIP variant on monocyte mRNA expression and M. tuberculosis-induced monocyte immune functions. We also examined the association of TOLLIP variation with BCG-induced T-cell responses and susceptibility to latent tuberculosis infection.
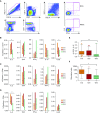
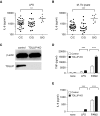
Measurements and main results: We identified a functional TOLLIP promoter region single-nucleotide polymorphism, rs5743854, which was associated with decreased TOLLIP mRNA expression in infant monocytes. After M. tuberculosis infection, TOLLIP-deficient monocytes demonstrated increased IL-6, increased nitrite, and decreased bacterial replication. The TOLLIP-deficiency G/G genotype was associated with decreased BCG-specific IL-2+ CD4+ T-cell frequency and proliferation. This genotype was also associated with increased susceptibility to latent tuberculosis infection.
Conclusions: TOLLIP deficiency is associated with decreased BCG-specific T-cell responses and increased susceptibility to tuberculosis. We hypothesize that the heightened antibacterial monocyte responses after vaccination of TOLLIP-deficient infants are responsible for decreased BCG-specific T-cell responses. Activating TOLLIP may provide a novel adjuvant strategy for BCG vaccination.
Keywords: Toll-interacting protein; adaptive immunity; bacillus Calmette-Guérin; genetics; tuberculosis.
Figures


References
-
- Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, Dansereau EA, Graetz N, Barber RM, Brown JC, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–1070. - PMC - PubMed
-
- Calmette A, Guerin C, Boquet A, Negre L. La vaccination preventive contre la tuberculose par le “BCG.”. Paris: Masson et cie; 1927.
-
- Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–480. - PubMed
-
- Cobat A, Hoal EG, Gallant CJ, Simkin L, Black GF, Stanley K, Jaïs JP, Yu TH, Boland-Auge A, Grange G, et al. Identification of a major locus, TNF1, that controls BCG-triggered tumor necrosis factor production by leukocytes in an area hyperendemic for tuberculosis. Clin Infect Dis. 2013;57:963–970. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

