Transcriptional circuits in B cell transformation
- PMID: 28463873
- PMCID: PMC5701664
- DOI: 10.1097/MOH.0000000000000352
Transcriptional circuits in B cell transformation
Abstract
Purpose of review: Loss of IKAROS in committed B cell precursors causes a block in differentiation while at the same time augments aberrant cellular properties, such as bone marrow stromal adhesion, self-renewal and resistance to glucocorticoid-mediated cell death. B cell acute lymphoblastic leukaemias originating from these early stages of B cell differentiation and associated with IKAROS mutations share a high-risk cellular phenotype suggesting that deregulation of IKAROS-based mechanisms cause a highly malignant disease process.
Recent studies: Recent studies show that IKAROS is critical for the activity of super-enhancers at genes required for pre-B cell receptor (BCR) signalling and differentiation, working either downstream of or in parallel with B cell master regulators such as EBF1 and PAX5. IKAROS also directly represses a cryptic regulatory network of transcription factors prevalent in mesenchymal and epithelial precursors that includes YAP1, TEAD1/2, LHX2 and LMO2, and their targets, which are not normally expressed in lymphocytes. IKAROS prevents not only expression of these 'extra-lineage' transcription factors but also their cooperation with endogenous B cell master regulators, such as EBF1 and PAX5, leading to the formation of a de novo for lymphocytes super-enhancer network. IKAROS coordinates with the Polycomb repression complex (PRC2) to provide stable repression of associated genes during B cell development. However, induction of regulatory factors normally repressed by IKAROS starts a feed-forward loop that activates de-novo enhancers and elevates them to super-enhancer status, thereby diminishing PRC2 repression and awakening aberrant epithelial-like cell properties in B cell precursors.
Summary: Insight into IKAROS-based transcriptional circuits not only sets new paradigms for cell differentiation but also provides new approaches for classifying and treating high-risk human B-ALL that originates from these early stages of B cell differentiation.
Conflict of interest statement
We declare no conflict of interest.
Figures
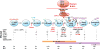


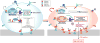
References
-
- Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9:195–205. - PubMed
-
- Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26:715–725. - PubMed
-
- Bryder D, Sigvardsson M. Shaping up a lineage--lessons from B lymphopoesis. Curr Opin Immunol. 2010;22:148–153. - PubMed
-
- Mandel EM, Grosschedl R. Transcription control of early B cell differentiation. Curr Opin Immunol. 2010;22:161–167. - PubMed
-
- Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds SB, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

