α-Klotho expression determines nitric oxide synthesis in response to FGF-23 in human aortic endothelial cells
- PMID: 28463984
- PMCID: PMC5413063
- DOI: 10.1371/journal.pone.0176817
α-Klotho expression determines nitric oxide synthesis in response to FGF-23 in human aortic endothelial cells
Abstract
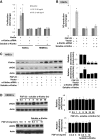
Endothelial cells (ECs) express fibroblast growth factor (FGF) receptors and are metabolically active after treatment with FGF-23. It is not known if this effect is α-Klotho independent or mediated by humoral or endogenous endothelial α-Klotho. In the present study, we aimed to characterize EC α-Klotho expression within the human vascular tree and to investigate the potential role of α-Klotho in determining FGF-23 mediated EC regulation. Human tissue and ECs from various organs were used for immunohistochemistry and Western blot. Primary cultures of human aortic endothelial cells (HAECs) and human brain microvascular endothelial cells (HBMECs) were used to generate in vitro cell models. We found endogenous α-Klotho expression in ECs from various organs except in microvascular ECs from human brain. Furthermore, FGF-23 stimulated endothelial nitric oxide synthase (eNOS) expression, nitric oxide (NO) production, and cell proliferation in HAECs. Interestingly, these effects were not observed in our HBMEC model in vitro. High phosphate treatment and endothelial α-Klotho knockdown mitigated FGF-23 mediated eNOS induction, NO production, and cell proliferation in HAECs. Rescue treatment with soluble α-Klotho did not reverse endothelial FGF-23 resistance caused by reduced or absent α-Klotho expression in HAECs. These novel observations provide evidence for differential α-Klotho functional expression in the human endothelium and its presence may play a role in determining the response to FGF-23 in the vascular tree. α-Klotho was not detected in cerebral microvascular ECs and its absence may render these cells nonresponsive to FGF-23.
Conflict of interest statement
Figures



References
-
- Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801 - DOI - PMC - PubMed
-
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002;287:2570–2581. - PubMed
-
- London GM, Pannier B, Agharazii M, Guerin AP, Verbeke FH, Marchais SJ. Forearm reactive hyperemia and mortality in end-stage renal disease. Kidney Int 2004;65:700–704. doi: 10.1111/j.1523-1755.2004.00434.x - DOI - PubMed
-
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006;444:770–774. doi: 10.1038/nature05315 - DOI - PubMed
-
- Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008;359:584–592. doi: 10.1056/NEJMoa0706130 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

