First-trimester artemisinin derivatives and quinine treatments and the risk of adverse pregnancy outcomes in Africa and Asia: A meta-analysis of observational studies
- PMID: 28463996
- PMCID: PMC5412992
- DOI: 10.1371/journal.pmed.1002290
First-trimester artemisinin derivatives and quinine treatments and the risk of adverse pregnancy outcomes in Africa and Asia: A meta-analysis of observational studies
Abstract
Background: Animal embryotoxicity data, and the scarcity of safety data in human pregnancies, have prevented artemisinin derivatives from being recommended for malaria treatment in the first trimester except in lifesaving circumstances. We conducted a meta-analysis of prospective observational studies comparing the risk of miscarriage, stillbirth, and major congenital anomaly (primary outcomes) among first-trimester pregnancies treated with artemisinin derivatives versus quinine or no antimalarial treatment.
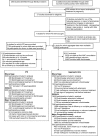
Methods and findings: Electronic databases including Medline, Embase, and Malaria in Pregnancy Library were searched, and investigators contacted. Five studies involving 30,618 pregnancies were included; four from sub-Saharan Africa (n = 6,666 pregnancies, six sites) and one from Thailand (n = 23,952). Antimalarial exposures were ascertained by self-report or active detection and confirmed by prescriptions, clinic cards, and outpatient registers. Cox proportional hazards models, accounting for time under observation and gestational age at enrollment, were used to calculate hazard ratios. Individual participant data (IPD) meta-analysis was used to combine the African studies, and the results were then combined with those from Thailand using aggregated data meta-analysis with a random effects model. There was no difference in the risk of miscarriage associated with the use of artemisinins anytime during the first trimester (n = 37/671) compared with quinine (n = 96/945; adjusted hazard ratio [aHR] = 0.73 [95% CI 0.44, 1.21], I2 = 0%, p = 0.228), in the risk of stillbirth (artemisinins, n = 10/654; quinine, n = 11/615; aHR = 0.29 [95% CI 0.08-1.02], p = 0.053), or in the risk of miscarriage and stillbirth combined (pregnancy loss) (aHR = 0.58 [95% CI 0.36-1.02], p = 0.099). The corresponding risks of miscarriage, stillbirth, and pregnancy loss in a sensitivity analysis restricted to artemisinin exposures during the embryo sensitive period (6-12 wk gestation) were as follows: aHR = 1.04 (95% CI 0.54-2.01), I2 = 0%, p = 0.910; aHR = 0.73 (95% CI 0.26-2.06), p = 0.551; and aHR = 0.98 (95% CI 0.52-2.04), p = 0.603. The prevalence of major congenital anomalies was similar for first-trimester artemisinin (1.5% [95% CI 0.6%-3.5%]) and quinine exposures (1.2% [95% CI 0.6%-2.4%]). Key limitations of the study include the inability to control for confounding by indication in the African studies, the paucity of data on potential confounders, the limited statistical power to detect differences in congenital anomalies, and the lack of assessment of cardiovascular defects in newborns.
Conclusions: Compared to quinine, artemisinin treatment in the first trimester was not associated with an increased risk of miscarriage or stillbirth. While the data are limited, they indicate no difference in the prevalence of major congenital anomalies between treatment groups. The benefits of 3-d artemisinin combination therapy regimens to treat malaria in early pregnancy are likely to outweigh the adverse outcomes of partially treated malaria, which can occur with oral quinine because of the known poor adherence to 7-d regimens.
Review registration: PROSPERO CRD42015032371.
Conflict of interest statement
Sigma Tau Industrie Farmaceutiche Riunite: AS is a co-investigator in a trial on Eurartesim dispersible in infants (6-12 months old) with uncomplicated malaria, and also received financial support for conference attendance. Medicine for Malaria Venture: AS is a member of the External Scientific Advisory Committee for the Medicine for Malaria Venture, which also provided financial support for conference attendance.
Figures


References
-
- World Health Organization. Guidelines for the treatment of malaria. Third edition Geneva: World Health Organization; 2015.
-
- Clark R, Kumemura M, Makori N, Nakata Y, Bernard F, Harrell A, et al. Artesunate: developmental toxicity in monkeys. Birth Defects Res A Clin Mol Teratol. 2006;76:329.
-
- Clark RL. Embryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimester. Reprod Toxicol. 2009;28(3):285–96. doi: 10.1016/j.reprotox.2009.05.002 - DOI - PubMed
-
- Clark RL, Arima A, Makori N, Nakata Y, Bernard F, Gristwood W, et al. Artesunate: developmental toxicity and toxicokinetics in monkeys. Birth Defects Res B Dev Reprod Toxicol. 2008;83(4):418–34. doi: 10.1002/bdrb.20163 - DOI - PubMed
-
- Clark RL, Lerman SA, Cox EM, Gristwood WE, White TE. Developmental toxicity of artesunate in the rat: comparison to other artemisinins, comparison of embryotoxicity and kinetics by oral and intravenous routes, and relationship to maternal reticulocyte count. Birth Defects Res B Dev Reprod Toxicol. 2008;83(4):397–406. doi: 10.1002/bdrb.20165 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

