A Long-Range Acting Dehydratase Domain as the Missing Link for C17-Dehydration in Iso-Migrastatin Biosynthesis
- PMID: 28464455
- PMCID: PMC5558199
- DOI: 10.1002/anie.201703588
A Long-Range Acting Dehydratase Domain as the Missing Link for C17-Dehydration in Iso-Migrastatin Biosynthesis
Abstract
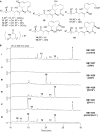
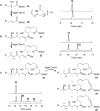
The dehydratase domains (DHs) of the iso-migrastatin (iso-MGS) polyketide synthase (PKS) were investigated by systematic inactivation of the DHs in module-6, -9, -10 of MgsF (i.e., DH6, DH9, DH10) and module-11 of MgsG (i.e., DH11) in vivo, followed by structural characterization of the metabolites accumulated by the mutants, and biochemical characterization of DH10 in vitro, using polyketide substrate mimics with varying chain lengths. These studies allowed us to assign the functions for all four DHs, identifying DH10 as the dedicated dehydratase that catalyzes the dehydration of the C17 hydroxy group during iso-MGS biosynthesis. In contrast to canonical DHs that catalyze dehydration of the β-hydroxy groups of the nascent polyketide intermediates, DH10 acts in a long-range manner that is unprecedented for type I PKSs, a novel dehydration mechanism that could be exploited for polyketide structural diversity by combinatorial biosynthesis and synthetic biology.
Keywords: biosynthesis; dehydratase; iso-migrastatin; natural products; polyketides.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Figures


Similar articles
-
Comparative characterization of the lactimidomycin and iso-migrastatin biosynthetic machineries revealing unusual features for acyltransferase-less type I polyketide synthases and providing an opportunity to engineer new analogues.Biochemistry. 2014 Dec 16;53(49):7854-65. doi: 10.1021/bi501396v. Epub 2014 Dec 1. Biochemistry. 2014. PMID: 25405956 Free PMC article.
-
Post-polyketide synthase steps in iso-migrastatin biosynthesis, featuring tailoring enzymes with broad substrate specificity.J Am Chem Soc. 2013 Feb 20;135(7):2489-92. doi: 10.1021/ja4002635. Epub 2013 Feb 11. J Am Chem Soc. 2013. PMID: 23394593 Free PMC article.
-
iso-Migrastatin, migrastatin, and dorrigocin production in Streptomyces platensis NRRL 18993 is governed by a single biosynthetic machinery featuring an acyltransferase-less type I polyketide synthase.J Biol Chem. 2009 Oct 23;284(43):29746-56. doi: 10.1074/jbc.M109.046805. Epub 2009 Sep 2. J Biol Chem. 2009. PMID: 19726666 Free PMC article.
-
Iterative polyketide biosynthesis by modular polyketide synthases in bacteria.Appl Microbiol Biotechnol. 2016 Jan;100(2):541-57. doi: 10.1007/s00253-015-7093-0. Epub 2015 Nov 9. Appl Microbiol Biotechnol. 2016. PMID: 26549236 Free PMC article. Review.
-
Insights into polyketide biosynthesis gained from repurposing antibiotic-producing polyketide synthases to produce fuels and chemicals.J Antibiot (Tokyo). 2016 Jul;69(7):494-9. doi: 10.1038/ja.2016.64. Epub 2016 Jun 1. J Antibiot (Tokyo). 2016. PMID: 27245558 Review.
Cited by
-
Programmed Iteration Controls the Assembly of the Nonanoic Acid Side Chain of the Antibiotic Mupirocin.Angew Chem Int Ed Engl. 2022 Dec 12;61(50):e202212393. doi: 10.1002/anie.202212393. Epub 2022 Nov 10. Angew Chem Int Ed Engl. 2022. PMID: 36227272 Free PMC article.
-
Loss of Single-Domain Function in a Modular Assembly Line Alters the Size and Shape of a Complex Polyketide.Angew Chem Int Ed Engl. 2019 Dec 9;58(50):18252-18256. doi: 10.1002/anie.201911315. Epub 2019 Oct 30. Angew Chem Int Ed Engl. 2019. PMID: 31595618 Free PMC article.
-
Programmed Iteration Controls the Assembly of the Nonanoic Acid Side Chain of the Antibiotic Mupirocin.Angew Chem Weinheim Bergstr Ger. 2022 Dec 12;134(50):e202212393. doi: 10.1002/ange.202212393. Epub 2022 Nov 10. Angew Chem Weinheim Bergstr Ger. 2022. PMID: 38505625 Free PMC article.
-
Discrete Acyltransferases and Thioesterases in Iso-Migrastatin and Lactimidomycin Biosynthesis.Biochemistry. 2024 Feb 12:10.1021/acs.biochem.3c00672. doi: 10.1021/acs.biochem.3c00672. Online ahead of print. Biochemistry. 2024. PMID: 38345531 Free PMC article.
-
Insights into azalomycin F assembly-line contribute to evolution-guided polyketide synthase engineering and identification of intermodular recognition.Nat Commun. 2023 Feb 4;14(1):612. doi: 10.1038/s41467-023-36213-9. Nat Commun. 2023. PMID: 36739290 Free PMC article.
References
-
- Woo EJ, Starks CM, Carney JR, Arslanian R, Cardapan L, Zavala S, Licari P. J Antibiot. 2002;55:141–146. - PubMed
-
- Sugawara K, Nishiyama Y, Toda S, Komiyama N, Hatori M, Moriyama T, Sawada Y, Kamei H, Konishi M, Oki T. J Antibiot. 1992;45:1433–1441. - PubMed
-
- Frohardt RP, Dion HW, Jakubowski ZL, Ryder A, French JC, Bartz QR. J Am Chem Soc. 1959;81:5500–5506.
-
- Saito N, Kitame F, Kikuchi M, Ishida N. J Antibiot. 1974;27:206–214. - PubMed
-
- Leach BE, Ford JH, Whiffen AJ. J Am Chem Soc. 1947;69:474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

