Pathogenesis of virulent and attenuated foot-and-mouth disease virus in cattle
- PMID: 28464897
- PMCID: PMC5414290
- DOI: 10.1186/s12985-017-0758-9
Pathogenesis of virulent and attenuated foot-and-mouth disease virus in cattle
Abstract
Background: Understanding the mechanisms of attenuation and virulence of foot-and-mouth disease virus (FMDV) in the natural host species is critical for development of next-generation countermeasures such as live-attenuated vaccines. Functional genomics analyses of FMDV have identified few virulence factors of which the leader proteinase (Lpro) is the most thoroughly investigated. Previous work from our laboratory has characterized host factors in cattle inoculated with virulent FMDV and attenuated mutant strains with transposon insertions within Lpro.
Methods: In the current study, the characteristics defining virulence of FMDV in cattle were further investigated by comparing the pathogenesis of a mutant, attenuated strain (FMDV-Mut) to the parental, virulent virus from which the mutant was derived (FMDV-WT). The only difference between the two viruses was an insertion mutation in the inter-AUG region of the leader proteinase of FMDV-Mut. All cattle were infected by simulated-natural, aerosol inoculation.
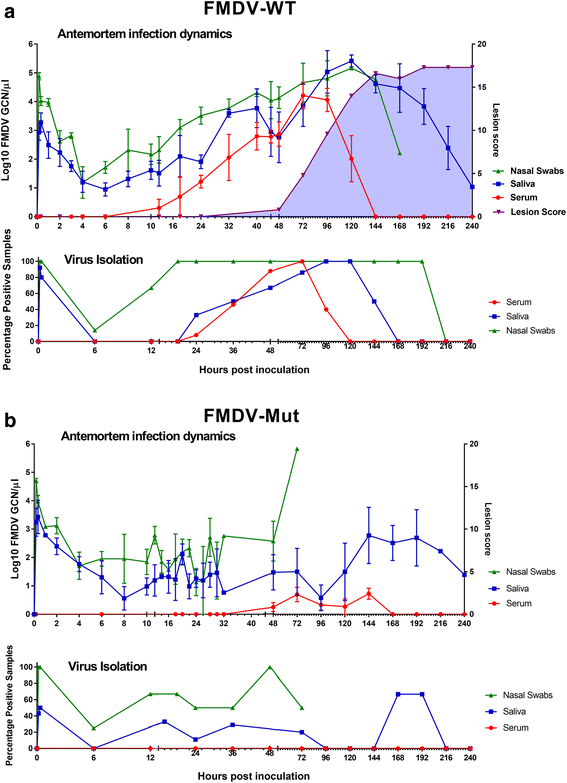
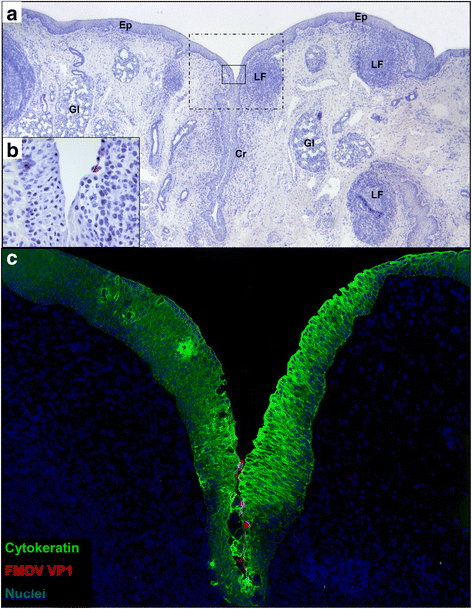
Results: Both viruses were demonstrated to establish primary infection in the nasopharyngeal mucosa with subsequent dissemination to the lungs. Immunomicroscopic localization of FMDV antigens indicated that both viruses infected superficial epithelial cells of the nasopharynx and lungs. The critical differences between the two viruses were a more rapid establishment of infection by FMDV-WT and quantitatively greater virus loads in secretions and infected tissues compared to FMDV-Mut. The slower replicating FMDV-Mut established a subclinical infection that was limited to respiratory epithelial sites, whereas the faster replication of FMDV-WT facilitated establishment of viremia, systemic dissemination of infection, and clinical disease.
Conclusion: The mutant FMDV was capable of achieving all the same early pathogenesis landmarks as FMDV-WT, but was unable to establish systemic infection. The precise mechanism of attenuation remains undetermined; but current data suggests that the impaired replication of the mutant is more responsible for attenuation than differences in host immunological factors. These results complement previous studies by providing data of high-granularity describing tissue-specific tropism of FMDV and by demonstrating microscopic localization of virulent and attenuated clones of the same field-strain FMDV.
Keywords: Bovine; Cattle; FMD; FMDV; Foot-and-mouth; Pathogenesis; Virulence; Virus.
Figures
References
-
- Arzt J, Pacheco JM, Smoliga GR, Tucker MT, Bishop E, Pauszek SJ, Hartwig EJ, de los Santos T, Rodriguez LL. Foot-and-mouth disease virus virulence in cattle is co-determined by viral replication dynamics and route of infection. Virology. 2014;452–453:12–22. doi: 10.1016/j.virol.2014.01.001. - DOI - PubMed
-
- Fowler V, Bashiruddin JB, Belsham GJ, Stenfeldt C, Botner A, Knowles NJ, Bankowski B, Parida S, Barnett P. Characteristics of a foot-and-mouth disease virus with a partial VP1 G-H loop deletion in experimentally infected cattle. Vet Microbiol. 2014;169(1–2):58–66. doi: 10.1016/j.vetmic.2013.12.008. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

