The biological function of the cellular prion protein: an update
- PMID: 28464931
- PMCID: PMC5412054
- DOI: 10.1186/s12915-017-0375-5
The biological function of the cellular prion protein: an update
Abstract
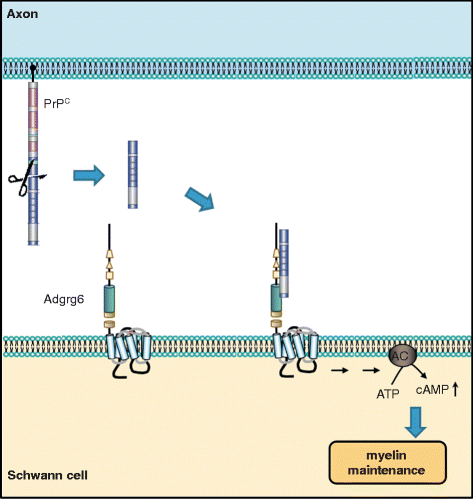
The misfolding of the cellular prion protein (PrPC) causes fatal neurodegenerative diseases. Yet PrPC is highly conserved in mammals, suggesting that it exerts beneficial functions preventing its evolutionary elimination. Ablation of PrPC in mice results in well-defined structural and functional alterations in the peripheral nervous system. Many additional phenotypes were ascribed to the lack of PrPC, but some of these were found to arise from genetic artifacts of the underlying mouse models. Here, we revisit the proposed physiological roles of PrPC in the central and peripheral nervous systems and highlight the need for their critical reassessment using new, rigorously controlled animal models.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

