Seasonal vaccination against malaria: a potential use for an imperfect malaria vaccine
- PMID: 28464937
- PMCID: PMC5414195
- DOI: 10.1186/s12936-017-1841-9
Seasonal vaccination against malaria: a potential use for an imperfect malaria vaccine
Abstract
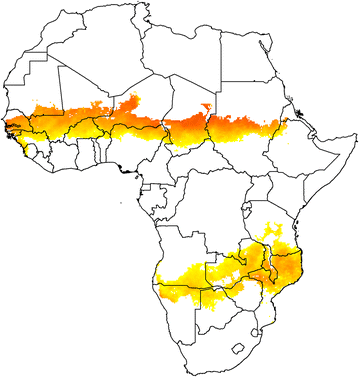
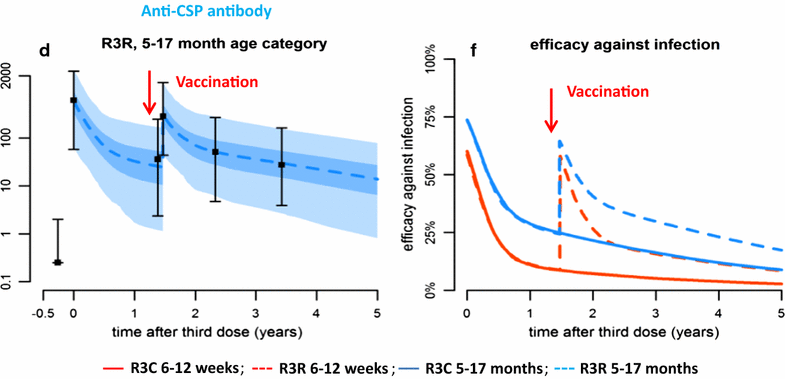
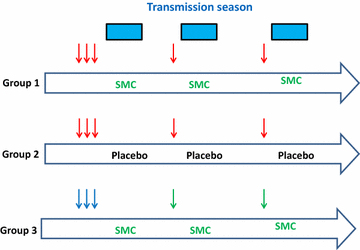
In many parts of the African Sahel and sub-Sahel, where malaria remains a major cause of mortality and morbidity, transmission of the infection is highly seasonal. Seasonal malaria chemoprevention (SMC), which involves administration of a full course of malaria treatment to young children at monthly intervals during the high transmission season, is proving to be an effective malaria control measure in these areas. However, SMC does not provide complete protection and it is demanding to deliver for both families and healthcare givers. Furthermore, there is a risk of the emergence in the future of resistance to the drugs, sulfadoxine-pyrimethamine and amodiaquine, that are currently being used for SMC. Substantial progress has been made in the development of malaria vaccines during the past decade and one malaria vaccine, RTS,S/AS01, has received a positive opinion from the European Medicines Authority and will soon be deployed in large-scale, pilot implementation projects in sub-Saharan Africa. A characteristic feature of this vaccine, and potentially of some of the other malaria vaccines under development, is that they provide a high level of efficacy during the period immediately after vaccination, but that this wanes rapidly, perhaps because it is difficult to develop effective immunological memory to malaria antigens in subjects exposed previously to malaria infection. A potentially effective way of using malaria vaccines with high initial efficacy but which provide only a short period of protection could be annual, mass vaccination campaigns shortly before each malaria transmission season in areas where malaria transmission is confined largely to a few months of the year.
Keywords: Seasonal malaria chemoprevention; Seasonal malaria transmission; Seasonal malaria vaccination.
Figures
References
-
- WHO. Policy recommendation: seasonal malaria chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. Geneva: World Health Organization; 2012. http://www.who.int/malaria/publications/atoz/who_smc_policy_recommendati.... Accessed 10 Feb 2017.
-
- http://www.malariaconsortium.org/what-we-do/projects/41/access-smc. Accessed 10 Feb 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

