Quiescence of human muscle stem cells is favored by culture on natural biopolymeric films
- PMID: 28464938
- PMCID: PMC5414338
- DOI: 10.1186/s13287-017-0556-8
Quiescence of human muscle stem cells is favored by culture on natural biopolymeric films
Abstract
Background: Satellite cells are quiescent resident muscle stem cells that present an important potential to regenerate damaged tissue. However, this potential is diminished once they are removed from their niche environment in vivo, prohibiting the long-term study and genetic investigation of these cells. This study therefore aimed to provide a novel biomaterial platform for the in-vitro culture of human satellite cells that maintains their stem-like quiescent state, an important step for cell therapeutic studies.
Methods: Human muscle satellite cells were isolated from two donors and cultured on soft biopolymeric films of controlled stiffness. Cell adhesive phenotype, maintenance of satellite cell quiescence and capacity for gene manipulation were investigated using FACS, western blotting, fluorescence microscopy and electron microscopy.
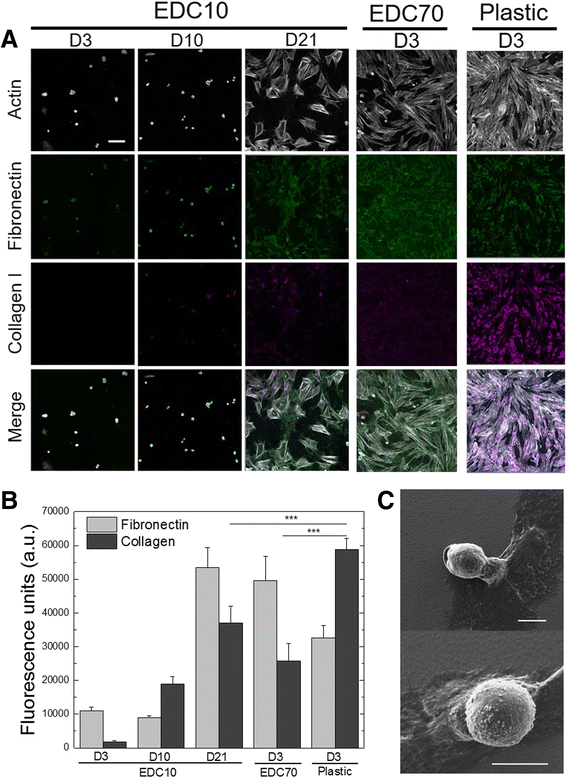
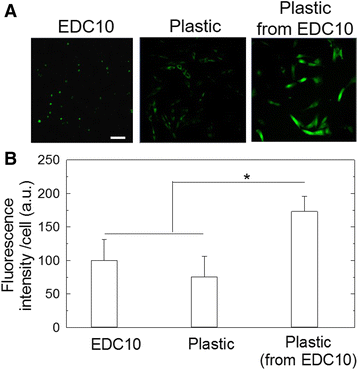
Results: About 85% of satellite cells cultured in vitro on soft biopolymer films for 3 days maintained expression of the quiescence marker Pax7, as compared with 60% on stiffer films and 50% on tissue culture plastic. The soft biopolymeric films allowed satellite cell culture for up to 6 days without renewing the media. These cells retained their stem-like properties, as evidenced by the expression of stem cell markers and reduced expression of differentiated markers. In addition, 95% of cells grown on these soft biopolymeric films were in the G0/G1 stage of the cell cycle, as opposed to those grown on plastic that became activated and began to proliferate and differentiate.
Conclusions: Our study identifies a new biomaterial made of a biopolymer thin film for the maintenance of the quiescence state of muscle satellite cells. These cells could be activated at any point simply by replating them onto a plastic culture dish. Furthermore, these cells could be genetically manipulated by viral transduction, showing that this biomaterial may be further used for therapeutic strategies.
Keywords: Biomimetism; Culture platform; Layer by layer; Polymeric biomaterial; Quiescence; Satellite cells.
Figures




References
-
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

