A multiple reader scoring system for Nasal Potential Difference parameters
- PMID: 28465124
- PMCID: PMC5671639
- DOI: 10.1016/j.jcf.2017.04.011
A multiple reader scoring system for Nasal Potential Difference parameters
Abstract
Background: Nasal Potential Difference (NPD) is a biomarker of CFTR activity used to diagnose CF and monitor experimental therapies. Limited studies have been performed to assess agreement between expert readers of NPD interpretation using a scoring algorithm.
Methods: We developed a standardized scoring algorithm for "interpretability" and "confidence" for PD (potential difference) measures, and sought to determine the degree of agreement on NPD parameters between trained readers.
Results: There was excellent agreement for interpretability between NPD readers for CF and fair agreement for normal tracings but slight agreement of interpretability in indeterminate tracings. Amongst interpretable tracings, excellent correlation of mean scores for Ringer's Baseline PD, Δamiloride, and ΔCl-free+Isoproterenol was observed. There was slight agreement regarding confidence of the interpretable PD tracings, resulting in divergence of the Ringers and Δamiloride, and ΔCl-free+Isoproterenol PDs between "high" and "low" confidence CF tracings.
Conclusion: A multi-reader process with adjudication is important for scoring NPDs for diagnosis and in monitoring of CF clinical trials.
Keywords: CFTR; Clinical trial outcomes; Nasal Potential Difference.
Copyright © 2017 European Cystic Fibrosis Society. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest
None.
Figures

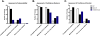
References
-
- Knowles MR, Carson JL, Collier AM, Gatzy JT, Boucher RC. Measurements of nasal transepithelial electric potential differences in normal human subjects in vivo. Am Rev Respir Dis. 1981;124(4):484–90. - PubMed
-
- Knowles MR, Buntin WH, Bromberg PA, Gatzy JT, Boucher RC. Measurements of transepithelial electric potential differences in the trachea and bronchi of human subjects in vivo. Am Rev Respir Dis. 1982;126(1):108–12. - PubMed
-
- Knowles MR, Stutts MJ, Yankaskas JR, Gatzy JT, Boucher RC., Jr Abnormal respiratory epithelial ion transport in cystic fibrosis. Clin Chest Med. 1986;7(2):285–97. - PubMed
-
- Standaert TA, Boitano L, Emerson J, Milgram LJ, Konstan MW, Hunter J, et al. Standardized procedure for measurement of nasal potential difference: an outcome measure in multicenter cystic fibrosis clinical trials. Pediatr Pulmonol. 2004;37(5):385–92. - PubMed
-
- Schuler D, Sermet-Gaudelus I, Wilschanski M, Ballmann M, Dechaux M, Edelman A, et al. Basic protocol for transepithelial nasal potential difference measurements. J Cyst Fibros. 2004;3(Suppl. 2):151–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

