High adherence to the 'Wise List' treatment recommendations in Stockholm: a 15-year retrospective review of a multifaceted approach promoting rational use of medicines
- PMID: 28465306
- PMCID: PMC5775463
- DOI: 10.1136/bmjopen-2016-014345
High adherence to the 'Wise List' treatment recommendations in Stockholm: a 15-year retrospective review of a multifaceted approach promoting rational use of medicines
Abstract
Objectives: To present the 'Wise List' (a formulary of essential medicines for primary and specialised care in Stockholm Healthcare Region) and assess adherence to the recommendations over a 15-year period.
Design: Retrospective analysis of all prescription data in the Stockholm Healthcare Region between 2000 and 2015 in relation to the Wise List recommendations during the same time period.
Setting: All outpatient care in the Stockholm Healthcare Region.
Participants: All prescribers in the Stockholm Healthcare Region.
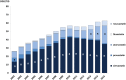
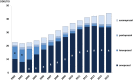
Main outcome measures: The number of core and complementary substances included in the Wise List, the adherence to recommendations by Anatomic Therapeutic Chemical (ATC) 1st level using defined daily doses (DDDs) adjusted to the DDD for 2015, adherence to recommendations over time measured by dispensed prescriptions yearly between 2002 and 2015.
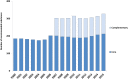
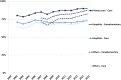
Results: The number of recommended core substances was stable (175-212). Overall adherence to the recommendations for core medicines for all prescribers increased from 75% to 84% (2000 to 2015). The adherence to recommendations in primary care for core medicines increased from 80% to 90% (2005 to 2015) with decreasing range in practice variation (32% to 13%). Hospital prescriber adherence to core medicine recommendations was stable but increased for the combination core and complementary medicines from 77% to 88% (2007 to 2015). Adherence varied between the 4 therapeutic areas studied.
Conclusions: High and increasing adherence to the Wise List recommendations was seen for all prescriber categories. The transparent process for developing recommendations involving respected experts and clinicians using strict criteria for handling potential conflicts of interests, feedback to prescribers, continuous medical education and financial incentives are possible contributing factors. High-quality evidence-based recommendations to prescribers, such as the Wise List, disseminated through a multifaceted approach, will become increasingly important and should be developed further to include recommendations and introduction protocols for new expensive medicines.
Keywords: Adherence; Drug and therapeutics committee; Essential medicines; Health systems; Rational Use of Medicines; prescribing.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: JE is the member of an expert panel of the Stockholm DTC since 2013. KA, PB-R, MJ-H, MJ, M-LO and BW are employed by Stockholm Healthcare Region that finances the Drug and Therapeutics Committee (DTC) issuing the ‘Wise List’ in Stockholm. EA-K served as the chair-woman of Stockholm DTC 2010–2016, LLG as the chairman 2000–2009 and GL is the chair-woman since 2016 and REM deputy chairman since 2016.
Figures
Similar articles
-
Primary care physicians report high trust in and usefulness of the Stockholm drug and therapeutic committee's list of recommended essential medicines (the 'Wise List').Eur J Clin Pharmacol. 2018 Jan;74(1):131-138. doi: 10.1007/s00228-017-2354-8. Epub 2017 Oct 23. Eur J Clin Pharmacol. 2018. PMID: 29063149 Free PMC article.
-
The 'wise list'- a comprehensive concept to select, communicate and achieve adherence to recommendations of essential drugs in ambulatory care in Stockholm.Basic Clin Pharmacol Toxicol. 2011 Apr;108(4):224-33. doi: 10.1111/j.1742-7843.2011.00682.x. Basic Clin Pharmacol Toxicol. 2011. PMID: 21414143
-
Evaluation of rational drug use based on World Health Organization core drug use indicators in selected public hospitals of eastern Ethiopia: a cross sectional study.BMC Health Serv Res. 2017 Feb 23;17(1):161. doi: 10.1186/s12913-017-2097-3. BMC Health Serv Res. 2017. PMID: 28231833 Free PMC article.
-
Prescribing indicators at primary health care centers within the WHO African region: a systematic analysis (1995-2015).BMC Public Health. 2016 Aug 22;16:724. doi: 10.1186/s12889-016-3428-8. BMC Public Health. 2016. PMID: 27545670 Free PMC article.
-
Inpatient drug utilization in Europe: nationwide data sources and a review of publications on a selected group of medicines (PROTECT project).Basic Clin Pharmacol Toxicol. 2015 Mar;116(3):201-11. doi: 10.1111/bcpt.12358. Epub 2014 Dec 29. Basic Clin Pharmacol Toxicol. 2015. PMID: 25420967 Review.
Cited by
-
Knowledge, attitudes and practices of healthcare professionals on the use of an electronic stock visibility and management tool in a middle-income country: Implications for access to medicines.Explor Res Clin Soc Pharm. 2023 Feb 1;9:100233. doi: 10.1016/j.rcsop.2023.100233. eCollection 2023 Mar. Explor Res Clin Soc Pharm. 2023. PMID: 36845673 Free PMC article.
-
Valproic acid utilization among girls and women in Stockholm: Impact of regulatory restrictions.Epilepsia Open. 2018 Jun 13;3(3):357-363. doi: 10.1002/epi4.12228. eCollection 2018 Sep. Epilepsia Open. 2018. PMID: 30187006 Free PMC article.
-
Sex differences in drugs: the development of a comprehensive knowledge base to improve gender awareness prescribing.Biol Sex Differ. 2017 Oct 24;8(1):32. doi: 10.1186/s13293-017-0155-5. Biol Sex Differ. 2017. PMID: 29065918 Free PMC article. No abstract available.
-
Acceptability of a short list of essential medicines to patients and prescribers: Multimethod study.Can Fam Physician. 2022 Jul;68(7):e204-e214. doi: 10.46747/cfp.6807e204. Can Fam Physician. 2022. PMID: 35831082 Free PMC article.
-
Different Policy Measures and Practices between Swedish Counties Influence Market Dynamics: Part 1-Biosimilar and Originator Infliximab in the Hospital Setting.BioDrugs. 2019 Jun;33(3):285-297. doi: 10.1007/s40259-019-00345-6. BioDrugs. 2019. PMID: 30945207 Free PMC article.
References
-
- WHO. The rational use of drugs—report of the conference of experts Nairobi, 25–29 November 1985. Geneva, Switzerland: World Health Organization, 1987. http://appswhoint/medicinedocs/documents/s17054e/s17054epdf
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
