Strenuous exercise triggers a life-threatening response in mice susceptible to malignant hyperthermia
- PMID: 28465322
- PMCID: PMC5503704
- DOI: 10.1096/fj.201601292R
Strenuous exercise triggers a life-threatening response in mice susceptible to malignant hyperthermia
Abstract
In humans, hyperthermic episodes can be triggered by halogenated anesthetics [malignant hyperthermia (MH) susceptibility] and by high temperature [environmental heat stroke (HS)]. Correlation between MH susceptibility and HS is supported by extensive work in mouse models that carry a mutation in ryanodine receptor type-1 (RYR1Y522S/WT) and calsequestrin-1 knockout (CASQ1-null), 2 proteins that control Ca2+ release in skeletal muscle. As overheating episodes in humans have also been described during exertion, here we subjected RYR1Y522S/WT and CASQ1-null mice to an exertional-stress protocol (incremental running on a treadmill at 34°C and 40% humidity). The mortality rate was 80 and 78.6% in RYR1Y522S/WT and CASQ1-null mice, respectively, vs. 0% in wild-type mice. Lethal crises were characterized by hyperthermia and rhabdomyolysis, classic features of MH episodes. Of importance, pretreatment with azumolene, an analog of the drug used in humans to treat MH crises, reduced mortality to 0 and 12.5% in RYR1Y522S/WT and CASQ1-null mice, respectively, thanks to a striking reduction of hyperthermia and rhabdomyolysis. At the molecular level, azumolene strongly prevented Ca2+-dependent activation of calpains and NF-κB by lowering myoplasmic Ca2+ concentration and nitro-oxidative stress, parameters that were elevated in RYR1Y522S/WT and CASQ1-null mice. These results suggest that common molecular mechanisms underlie MH crises and exertional HS in mice.-Michelucci, A., Paolini, C., Boncompagni, S., Canato, M., Reggiani, C., Protasi, F. Strenuous exercise triggers a life-threatening response in mice susceptible to malignant hyperthermia.
Keywords: calsequestrin-1; excitation-contraction coupling; ryanodine receptor; sarcoplasmic reticulum; skeletal muscle.
© FASEB.
Figures


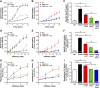
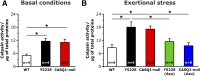



References
-
- Denborough M. A., Forster J. F., Lovell R. R., Maplestone P. A., Villiers J. D. (1962) Anaesthetic deaths in a family. Br. J. Anaesth. 34, 395–396 - PubMed
-
- MacLennan D. H., Phillips M. S. (1992) Malignant hyperthermia. Science 256, 789–794 - PubMed
-
- Wu S., Ibarra M. C., Malicdan M. C., Murayama K., Ichihara Y., Kikuchi H., Nonaka I., Noguchi S., Hayashi Y. K., Nishino I. (2006) Central core disease is due to RYR1 mutations in more than 90% of patients. Brain 129, 1470–1480 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

