13-Series resolvins mediate the leukocyte-platelet actions of atorvastatin and pravastatin in inflammatory arthritis
- PMID: 28465323
- PMCID: PMC5503705
- DOI: 10.1096/fj.201700268
13-Series resolvins mediate the leukocyte-platelet actions of atorvastatin and pravastatin in inflammatory arthritis
Abstract
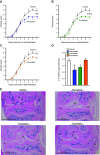
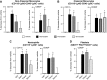
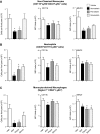
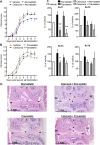
Rheumatoid arthritis is an inflammatory condition characterized by overzealous inflammation that leads to joint damage and is associated with an increased incidence of cardiovascular disease. Statins are frontline therapeutics for patients with cardiovascular disease and exert beneficial actions in rheumatoid arthritis. The mechanism that mediates the beneficial actions of statins in rheumatoid arthritis remains of interest. In the present study, we found that the administration of 2 clinically relevant statins-atorvastatin (0.2 mg/kg) or pravastatin (0.2 mg/kg)-to mice during inflammatory arthritis up-regulated systemic and tissue amounts of a novel family of proresolving mediators, termed 13-series resolvins (RvTs), and significantly reduced joint disease. Of note, administration of simvastatin (0.2 mg/kg) did not significantly up-regulate RvTs or reduce joint inflammation. We also found that atorvastatin and pravastatin each reduced systemic leukocyte activation, including platelet-monocyte aggregates (∼25-60%). These statins decreased neutrophil trafficking to the joint as well as joint monocyte and macrophage numbers. Atorvastatin and pravastatin produced significant reductions (∼30-50%) in expression of CD11b and major histocompatibility complex class II on both monocytes and monocyte-derived macrophages in joints. Administration of an inhibitor to cyclooxygenase-2, the initiating enzyme in the RvT pathway, reversed the protective actions of these statins on both joint and systemic inflammation. Together, these findings provide evidence for the role of RvTs in mediating the protective actions of atorvastatin and pravastatin in reducing local and vascular inflammation, and suggest that RvTs may be useful in measuring the anti-inflammatory actions of statins.-Walker, M. E., Souza, P. R., Colas, R. A., Dalli, J. 13-Series resolvins mediate the leukocyte-platelet actions of atorvastatin and pravastatin in inflammatory arthritis.
Keywords: eicosanoids; pharmacology; resolution; vascular inflammation; ω-3.
© The Author(s).
Figures

References
-
- Barra L. J., Pope J. E., Hitchon C., Boire G., Schieir O., Lin D., Thorne C. J., Tin D., Keystone E. C., Haraoui B., Jamal S., Bykerk V. P.; CATCH group (2017) The effect of rheumatoid arthritis-associated autoantibodies on the incidence of cardiovascular events in a large inception cohort of early inflammatory arthritis. [E-pub ahead of print] Rheumatology (Oxford) doi: 10.1093/rheumatology/kew474 - PubMed
-
- Giles J. T. (2015) Cardiovascular disease in rheumatoid arthritis: current perspectives on assessing and mitigating risk in clinical practice. Best Pract. Res. Clin. Rheumatol. 29, 597–613 - PubMed
-
- Taylor F. C., Huffman M., Ebrahim S. (2013) Statin therapy for primary prevention of cardiovascular disease. JAMA 310, 2451–2452 - PubMed
-
- Akiyama M., Mawatari T., Nakashima Y., Miyahara H., Yamada H., Okazaki K., Fukushi J., Kondo M., Kishimoto J., Hashimura C., Iwamoto Y. (2015) Prevalence of dyslipidemia in Japanese patients with rheumatoid arthritis and effects of atorvastatin treatment. Clin. Rheumatol. 34, 1867–1875 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

